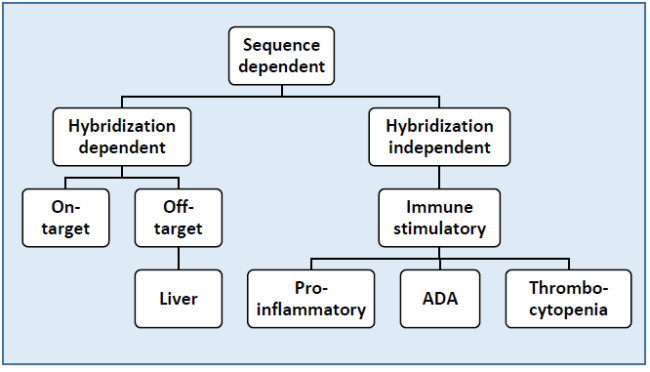
Description: Successful therapeutics need to be efficacious and safe. Although oligonucleotide therapeutics act via unique mechanisms, they follow the same principles for safety assessment as other modalities. This webinar will give an overview of these principles and what needs to be considered from a safety perspective when bringing oligonucleotide candidates to the clinic. The most common safety findings, known mechanisms and how these are affected by chemistry and design will be also be discussed.
Recording of the webinar: Click play to view
Presenter Biography

Patrik Andersson, PhD, ERT
Principal Scientist and RNA Therapeutics Safety Lead, Clinical Pharmacology and Safety Sciences, R&D, AstraZeneca Gothenburg
Patrik received 2003 a PhD in Toxicology at Karolinska Institutet, Stockholm, Sweden, followed by a short post-doc period at the Department of Medicinal Chemistry, Gothenburg University. In 2004, he joined AstraZeneca, Gothenburg, Sweden in the role of Discovery Toxicologist, providing preclinical safety support to a large number of projects in the Discovery phase. Since 2012, Patrik has focused on the safety aspects of nucleotide-based therapeutics, mainly antisense oligonucleotides, anti-microRNA and mRNA therapeutics. In 2014-2015 he spent twelve months on an industry sabbatical at AstraZeneca’s collaborators Ionis Pharmaceuticals and Regulus Therapeutics, supporting joint projects and learning about their oligo platforms. Back in Sweden, he has been the preclinical safety lead for ASO projects and platform activities including safety aspects of targeted delivery via different conjugation strategies.






