Presenter: Anastasia Khvorova, PhD, RNA Therapeutics Institute, University of Massachusetts Medical School
Date: 10 September 2018
Description:
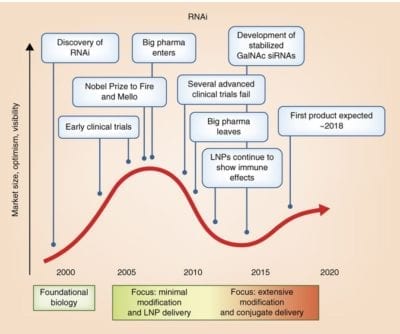
A decade of progress in oligonucleotide chemistry and formulation development has resulted in several compounds, both siRNAs and antisense, demonstrating robust clinical activity. Based on sequence, these types of drugs can be designed to modulate the expression of any disease-causing gene.
Clinical utility is defined by the ability to deliver to the tissue of interest. The most promising approach is modulation of oligonucleotide delivery through direct chemical modification. Full chemical stabilization, identification of optimal conjugates (GalNAc and others), and understanding the relationship between structure and pharmacokinetic/pharmacodynamic behavior are essential to enabling sustained (many months) efficacy following a single administration.
Recording of the Webinar: Click Play to View
Presenter Biography

Anastasia Khvorova, PhD,
RNA Therapeutics Institute, University of Massachusetts Medical School
Anastasia Khvorova, PhD, is Professor in the RNA Therapeutics Institute and the Program in Molecular Medicine at the University of Massachusetts Medical School (UMMS) in Worcester, Massachusetts. Before joining the UMMS faculty, she held leadership positions in industry, including VP of Research & Development and CSO of Dharmacon (now a Thermo Fisher Scientific company), Founder and Scientific Advisor of Advirna, and CSO and SVP of RXi Pharmaceuticals. Dr Khvorova is credited with several major advances in the field of RNA interference. Her team at Dharmacon developed and facilitated the commercialization of the first functional siRNA collection targeting the human genome (siGENOME), and at RXi, her group made significant progress in chemically modifying RNAi compounds to create self-delivering rxRNA compounds.
Dr. Khvorova’s industry experience in drug discovery and development collaborations with pharmaceutical companies along with her expertise in chemistry and cell biology allowed her to establish a lab at UMMS that brings together organic chemists and RNA biologists to develop novel approaches and solutions to understanding natural and therapeutic RNA trafficking and delivery. Dr. Khvorova is a member of the OTS Board of Directors and the editorial board of Nucleic Acid Research. She is the author of more than 150 abstracts, more than 50 peer-reviewed articles, several book chapters, and more than 250 patents and patent applications.






