
Patient-Customized Oligonucleotide Therapy for a Rare Genetic Disease
Kim J, Hu C, Moufawad El Achkar C, Black LE, Douville J, Larson A, Pendergast MK, Goldkind SF, Lee EA, Kuniholm A, Soucy A, Vaze J, Belur NR, Fredriksen K, Stojkovska I, Tsytsykova A, Armant M, DiDonato RL, Choi J, Cornelissen L, Pereira LM, Augustine EF, Genetti CA, Dies K, Barton B, Williams L, Goodlett BD, Riley BL, Pasternak A, Berry ER, Pflock KA, Chu S, Reed C, Tyndall K, Agrawal PB, Beggs AH, Grant PE, Urion DK, Snyder RO, Waisbren SE, Poduri A, Park PJ, Patterson A, Biffi A, Mazzulli JR, Bodamer O, Berde CB, Yu TW
Joint First Authors
Dr. Zhou Han
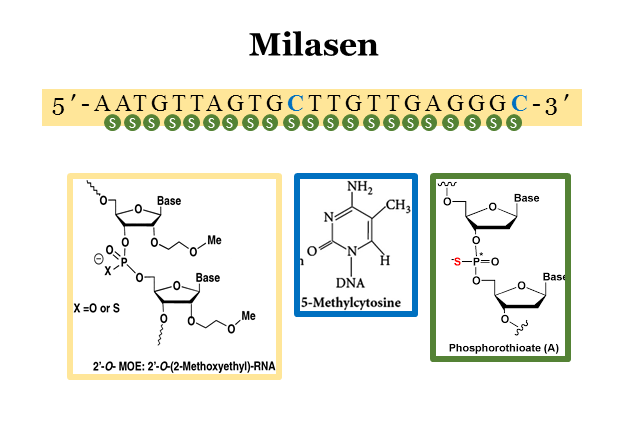
Using next generation sequencing, Kim, Hu and colleagues identified a heterozygous SVA retrotransposon integration in addition to a heterozygous c.1102G→C mutation in the MFSD8 gene of a patient with neuronal ceroid lipofuscinosis 7 (CLN7), an autosomal recessive form of Batten’s disease. The SVA retrotransposon integration activates a cryptic splice site acceptor in MFSD8 intron 6, causing mis-splicing and subsequent premature translation termination. Within the unprecedented short time of one year, the authors developed, tested, and treated the patient with a customized splice-switching oligonucleotide.
For more background see this video:
In patient fibroblasts, treatment with the 22-nt phosphorothioate, 2′-O-methoxyethyl antisense oligonucleotide Milasen increased correct splicing of the exon 6-7 junction around threefold, reaching levels found in the patient’s father, a carrier of the c.1102G→C mutation suggesting sufficient efficacy. Treatment also improved cellular phenotypes characteristic of the disease such as intracellular vacuolation, lysosomal mass, autofluorescence, non-lysosomal CGase, and reduced autophagic flux. Safety testing in rats showed no adverse events at 0.06 mg (equivalent to 2.5x of the typical clinical nusinersen dose).
Clinical investigational treatment under an Expanded Access Investigational New Drug application was started with an initial dose escalation period (from 3.5 mg to 42 mg, increasing every 2 weeks) and maintained with 42 mg every three months. No serious adverse events occurred. Despite dose dependent drug increases in cerebrospinal fluid, brain volume loss progressed. However, neurologic and neuropsychological measurements appeared to stabilize, and seizure frequency and duration decreased by more than 50%.
Why you should read it

Timothy Yu
The authors have realised the promise of personalized medicine with the rapid development of a mutation-specific treatment for their patient. Mila is only the first of many – see Lydia and the A-T Children’s Project for more!






