
Targeting Huntingtin Expression in Patients with Huntington’s Disease
Tabrizi SJ, Leavitt BR, Landwehrmeyer GB, Wild EJ, Saft C, Barker RA, Blair NF, Craufurd D, Priller J, Rickards H, Rosser A, Kordasiewicz HB, Czech C, Swayze EE, Norris DA, Baumann T, Gerlach I, Schobel SA, Paz E, Smith AV, Bennett CF, Lane RM.

Sarah Tabrizi

HTT research and clinical development team at Ionis
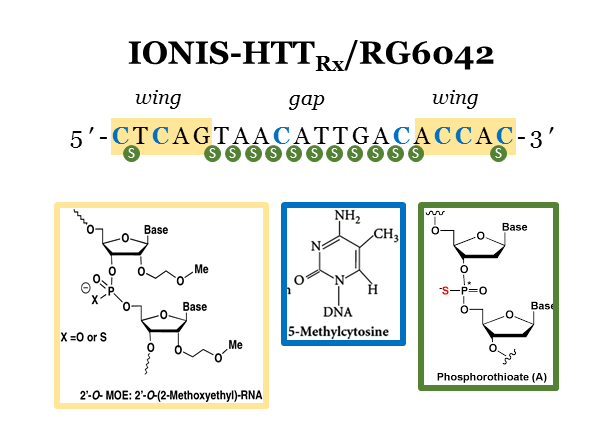
The success of nusinersen (Spinraza) has demonstrated that intrathecal delivery of antisense oligonucleotides can lead to long-term therapeutic benefits in the brain and established neurological diseases as priority targets for nucleic acid therapies. Here, Tabrizi and colleagues from the UK, Canada, and Germany together with teams from Ionis Pharmaceuticals and Roche report the results from a phase I/IIa trial (NCT02519036) using a RNaseH-based antisense oligonucleotide to down-regulate huntingtin expression.
Listen to Scott Schobel, Clinical Science Leader for the IONIS/Roche HTTRx program discussing the background of the trial:
Or watch Prof Tabrizi explaining the results in detail here:
An excellent summary written by Jerry Zon is available here and extended results up to 9 months from an ongoing OLE (NCT03342053) can be found here (slides 69-73).
In this safety and PK/PD study, 46 patients with early-stage Huntington’s disease received four intrathecal injections at 4-week intervals. Treatment was well tolerated, with only mild to moderate adverse events associated with the lumbar puncture procedure and no serious adverse events. There was no dose-limiting toxicity and all patients completed the study.
The concentration of therapeutic oligonucleotide increased in proportion to the dose in both plasma and cerebrospinal fluid (CSF) with no evidence of accumulation over time. Levels of mutant huntingtin in CSF decreased concurrently by 20-42% with steady-state maximal reduction not reached.
An initial analysis did not show any functional, cognitive, psychiatric, or neurologic differences in clinical outcomes correlating with huntingtin reductions. However, an exploratory post-hoc analysis using the composite Unified Huntington Disease Rating Scale (cUHDRS) offered some tentative evidence for therapeutic efficacy.
Why you should read it
This clinical trial provides proof of principle for lowering mutant huntingtin in the CNS and is the foundation for the ongoing phase III trial (NCT03761849).






