
March 2020 Joint Papers of the Month
Inclisiran for the Treatment of Heterozygous Familial Hypercholesterolemia.
Raal FJ, Kallend D, Ray KK, Turner T, Koenig W, Wright RS, Wijngaard PLJ, Curcio D, Jaros MJ, Leiter LA, Kastelein JJP; ORION-9 Investigators.

Prof. Frederick J. Raal
Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol.
Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, Bisch JA, Richardson T, Jaros M, Wijngaard PLJ, Kastelein JJP; ORION-10 and ORION-11 Investigators.

Prof. Kausic K. Ray
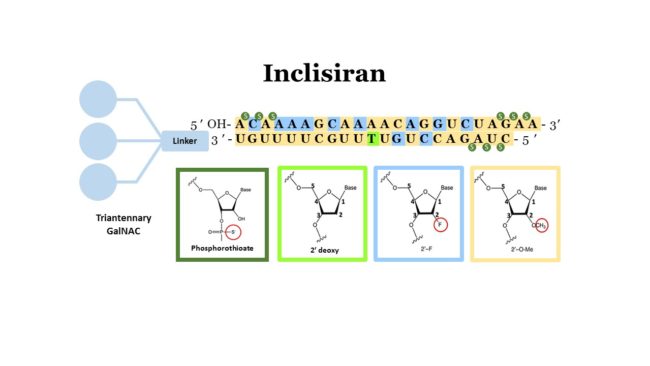
Inclisiran is a siRNA therapeutic targeting Proprotein convertase subtilisin/kexin type 9 (PCSK9), a protein responsible for degradation of Low-Density Lipoprotein Receptor (LDL-R) in the liver. Inhibition of PCSK9 results in an increase in LDL-R recycling after LDL unloading and therefore reduces LDL cholesterol levels in the blood. Early results of this oligonucleotide therapeutic were extremely promising (see here).
These two papers now report the results from phase III trials in patients with heterozygous familial hypercholesterolemia (ORION-9; NCT03397121), atherosclerotic cardiovascular disease (ORION-10; NCT03399370) or with elevated LDL cholesterol (ORION-11; NCT03400800) despite maximally tolerated statin therapy (with or without ezetimibe).
All patients in the treatment arms received 284 mg inclisiran via subcutaneous injection on days 1, 90, 270, and 450. Reductions in LDL cholesterol levels compared to the placebo group were statistically significant and consistent between trials (−47.9 % ORION-9; −52.3 % ORION-10; −49.9 % ORION-11). Inclisiran treatment was safe and well tolerated with the incidence of adverse events similar across groups. There was a slight increase in injection site reactions in the treatment groups in ORION-10 and 11 (1.5% and 4.2%) but curiously this percentage was 15.3% in the ORION-9 trial. All injection site reactions were mild to moderate.
To learn more about these outcomes, watch these investigators discuss the results in more detail:
Prof Frederick Raal on ORION-9
Prof R. Scott Wright on ORION-10
Prof Kausik K Ray on ORION-11
Other inclisiran trials such as the cardiovascular outcomes study (ORION-4; NCT03705234) are still ongoing. A primary prevention trial in collaboration with NHS UK is planned.
Inclisiran’s twice yearly administration schedule offers a clear advantage over currently available LDL-lowering therapies which suffer from non-compliance issues. Depending on pricing, a twice-yearly inclisiran shot may become standard of care for a large part of the population.
Why you should read it
Inclisiran is a triumph for siRNA therapeutics – it will be the most widely prescribed oligonucleotide therapy.






