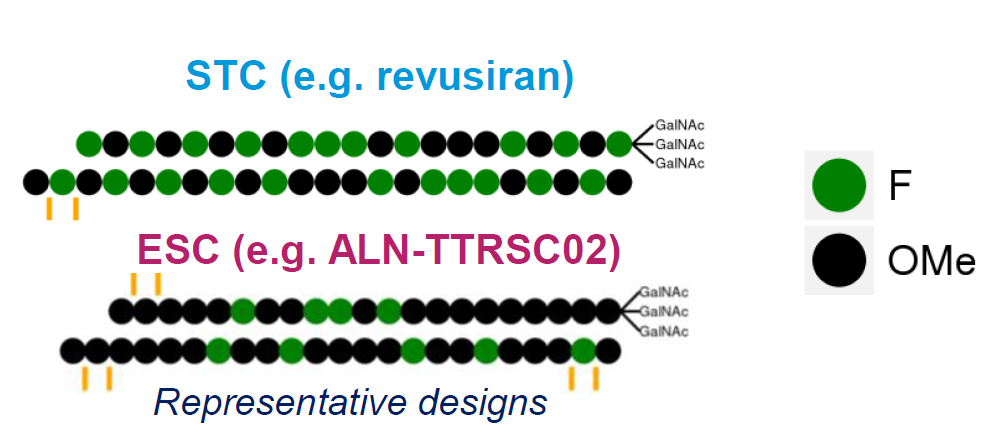

Safety evaluation of 2′-deoxy-2′-fluoro nucleotides in GalNAc-siRNA conjugates
Janas MM, Zlatev I, Liu J, Jiang Y, Barros SA, Sutherland JE, Davis WP, Liu J, Brown CR, Liu X, Schlegel MK, Blair L, Zhang X, Das B, Tran C, Aluri K, Li J, Agarwal S, Indrakanti R, Charisse K, Nair J, Matsuda S, Rajeev KG, Zimmermann T, Sepp-Lorenzino L, Xu Y, Akinc A, Fitzgerald K, Vaishnaw AK, Smith PF, Manoharan M, Jadhav V, Wu JT, Maier MA.
Nucleic Acids Res. 2019 Apr 23;47(7):3306-3320.

Chemical modifications of oligonucleotide therapeutics significantly improve their pharmacological and therapeutic properties. The 2′-F-modification is predominantly used in siRNA and aptamer therapeutics, but has been investigated in RNaseH and splice switching ASOs where some safety concerns have been raised.
To address these concerns, Janas, Zlatev et al. assess the impact of 2′F nucleoside metabolites (mono-, di- and tri-phosphate) on cellular and molecular processes.
Click here to download Janas’ presentation at OTS 2018 on this subject or watch the video for details of oligonucleotide chemistry:
The authors characterized 2′-F-monomer concentrations in rats after a single suprapharmacological dose. For revusiran (containing 22x 2′F-modifications), various 2′-F-monomers were detected in liver, kidney, plasma, heart and urine, all at low (<12.8) micromolar levels. Around 20% of total dose was excreted in urine. For the ESC (enhanced stability chemistry) ALN-TTRSC02 (9x 2′F-modifications), only 2′-F-purines were detectable and these solely in the liver. These concentrations are well below endogenous ribo- and deoxyribonucleotide levels. Chronic dosing did not result in accumulation.
In healthy human volunteers, only 2′-F-U and -I were detectable in plasma after 7.5 mg/kg daily for 5 days of revusiran (but not ALN-TTRSC02). Concentrations reached steady state within that period, suggesting that plasma half-life of these monomers is <1 day. Renal excretion was mainly in the form of oligomers. No 2′-F monomers were detectable in plasma or urine after a 50 mg dose of ALNTTRSC02.
For all 2′-F-NTPs, concentrations resulting in 50% (IC50) or 90% inhibition (IC90) of human cellular polymerases were above 200 µM, a 4:1 excess over native dNTPs. 2′-F-monomers were only incorporated into nascent mitochondrial DNA or RNA if present in significant excess relative to endogenous dNTPs and were unable to act as chain terminators during elongation. Some 2′-F-monomers had modest effects on cell viability and mitochondrial DNA at cellular concentrations above those reached during treatment in a subset of tested cell lines. A 2-year carcinogenicity study with weekly doses of up to 100 mg/kg in rats did not show differences in carcinogenicity, survival, or plasma lactate concentrations (a measure of mitochondrial toxicity).
Why you should read it
This paper demonstrates the safety of the 2′-F-modification widely used in aptamers and siRNA.






