
Traceless aptamer-mediated isolation of CD8+ T cells for chimeric antigen receptor T-cell therapy

Nataly Kacherovsky and Ian Cardle
Kacherovsky N, Cardle II, Cheng EL, Yu JL, Baldwin ML, Salipante SJ, Jensen MC, Pun SH.
Genetically engineered chimeric antigen receptor (CAR) T-cell therapies for liquid cancers have shown excellent results in clinical trials and two were approved by the FDA for clinical use in 2017 (Kymriah and Yescarta). However, purification of CD4+ and/or CD8+ T cells from the patient’s own blood relies on antibody-mediated selection using magnetic beads. This contributes to the significant production cost and imposes several practical limitations on the process. For example, antibody-coated magnetic beads remaining on the isolated T cells are a safety concern and prevent any further selection of subsets.
For a quick refresher on CAR T-cell therapy watch
For for a more detailed scientific explanation see Dr. Sarah Larson’s lecture:
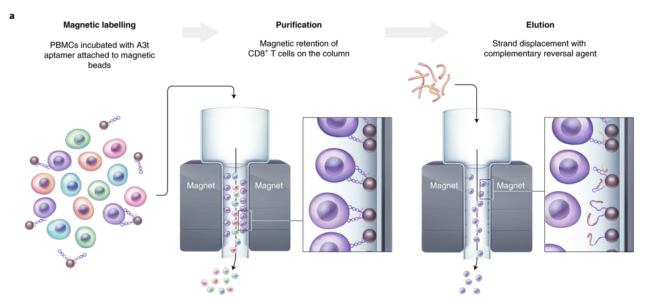
Kacherovsky and Cardle report on the isolation of DNA-aptamers with low-nanomolar affinity for CD8 using a modified cell−SELEX procedure that includes competitive selection with PBMCs and counter selection with a CD8− human T-cell leukaemia line. Specific binding to CD8 was confirmed by competitive binding with an antibody, siRNA-mediated CD8a downregulation, exogenous CD8a expression in CD8- cells and by biolayer interferometry as well as flow cytometry.
To enable isolation of label-free T cells, the authors designed a 36-base complementary oligonucleotide that disrupts aptamer folding and resulted in >90% aptamer release. When comparing aptamer immobilized on anti-biotin microbeads to the clinically approved CD8 antibody microbead method, CD8+ T cells could be isolated with similar purity, phenotype and functionality, though yields were decreased by 25% in the samples treated with aptamer reversal agent. CAR T cells generated from the aptamer- and antibody-isolated T cells showed comparable function both in vitro and in vivo.
Why you should read it
With an estimated price of ~10 US$ per clinical scale isolation, this method offers significant savings over antibody-based T-cell selection.

Michael Jensen







