
Allele-selective transcriptional repression of mutant HTT for the treatment of Huntington’s disease
Zeitler B, Froelich S, Marlen K, Shivak DA, Yu Q, Li D, Pearl JR, Miller JC, Zhang L, Paschon DE, Hinkley SJ, Ankoudinova I, Lam S, Guschin D, Kopan L, Cherone JM, Nguyen HB, Qiao G, Ataei Y, Mendel MC, Amora R, Surosky R, Laganiere J, Vu BJ, Narayanan A, Sedaghat Y, Tillack K, Thiede C, Gärtner A, Kwak S, Bard J, Mrzljak L, Park L, Heikkinen T, Lehtimäki KK, Svedberg MM, Häggkvist J, Tari L, Tóth M, Varrone A, Halldin C, Kudwa AE, Ramboz S, Day M, Kondapalli J, Surmeier DJ, Urnov FD, Gregory PD, Rebar EJ, Muñoz-Sanjuán I, Zhang HS.

Bryan Zeitler
Huntington’s disease (HD) is a neurodegenerative disorder caused by a CAG trinucleotide expansion in the huntingtin gene (HTT). Zinc finger protein transcription factors (ZFP-TFs) could be a great therapeutic strategy for HD as they inhibit all (potentially toxic) RNAs transcribed from their specific target DNA.
For background on engineered zinc finger proteins, watch this lecture by Sir Aaron Klug:
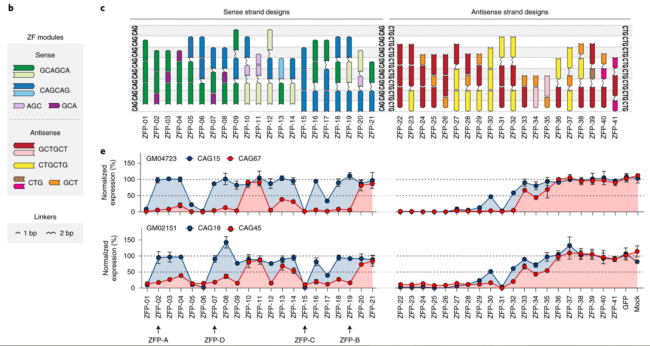
Zeitler and colleagues from Sangamo Therapeutics, Evotec AG, the CHDI Foundation and others report on the development of allele-selective ZFP-TFs that downregulate mHTT expression. The authors screened 41 poly-CAG-targeted ZFPs linked to the KRAB transcriptional repression domain in patient fibroblasts containing heterozygous pathogenic CAG repeat expansions. ZFP-A and ZFP-B were able to reduce expression from the CAG-repeat expanded HTT allele by >90% in a dose-dependent manner with minimal effect on the normal allele.
Using lentiviral delivery, suppression of mHTT was still effective at 74 days in neural stem cells (NSCs) and 103 days in neurons differentiated from CAG17/48 embryonic stem cells. Bilateral intrastriatal injection of AAV encoding ZFP-B in three mouse models resulted in up to 70% reduction in expression of the mHTT allele, with no effect on the endogenous mouse Htt. Reduced in vivo efficacy was congruent with limited levels of AAV striatal coverage (30–70%). Treatment led to improvement in locomotor, neuropathological and electrophysiological deficits as well as disease-associated histopathology. There was no evidence of treatment-associated neurodegeneration, neuroinflammation or toxicity. The ZFP-TFs suppressed a small number of other CAG repeat containing genes, with a distinct off-target profile for each ZFP-TF. This suggests that the off-target effects are design-dependent, and that continued optimization may be able to reduce or eliminate these. See Miller 2019 for more information on improved ZFP design.
Why you should read it
Optimized ZFP-TF design can target the HD associated CAG repeat expansion with high specificity.






