Looking for something?
Facts about COVID-19 mRNA Vaccines and the Decades of Research That Went into Creating Them
Posted: February 11, 2021 | Updated: October 8, 2021
Despite the global threat of COVID-19, mRNA vaccines, which are highly effective at preventing COVID-19 illness and have passed rigorous safety standards, have been met with skepticism by many people. This suspicion is due in part to misconceptions and misinformation that is circulating regarding mRNA vaccines. In this article, we are providing facts and scientific evidence to answer some of the most common questions and concerns.
Why are these called genetic vaccines? Does this mean the vaccine can change your DNA?
Both DNA and mRNA sequences are genetic material, which is why an mRNA vaccine is considered a genetic vaccine. However, DNA and mRNA operate under very specific rules and processes, and mRNA vaccines absolutely cannot change your DNA (1). Here is a simplified explanation of the intricate processes that actually take place to help you understand why your DNA is safe from an mRNA vaccine.
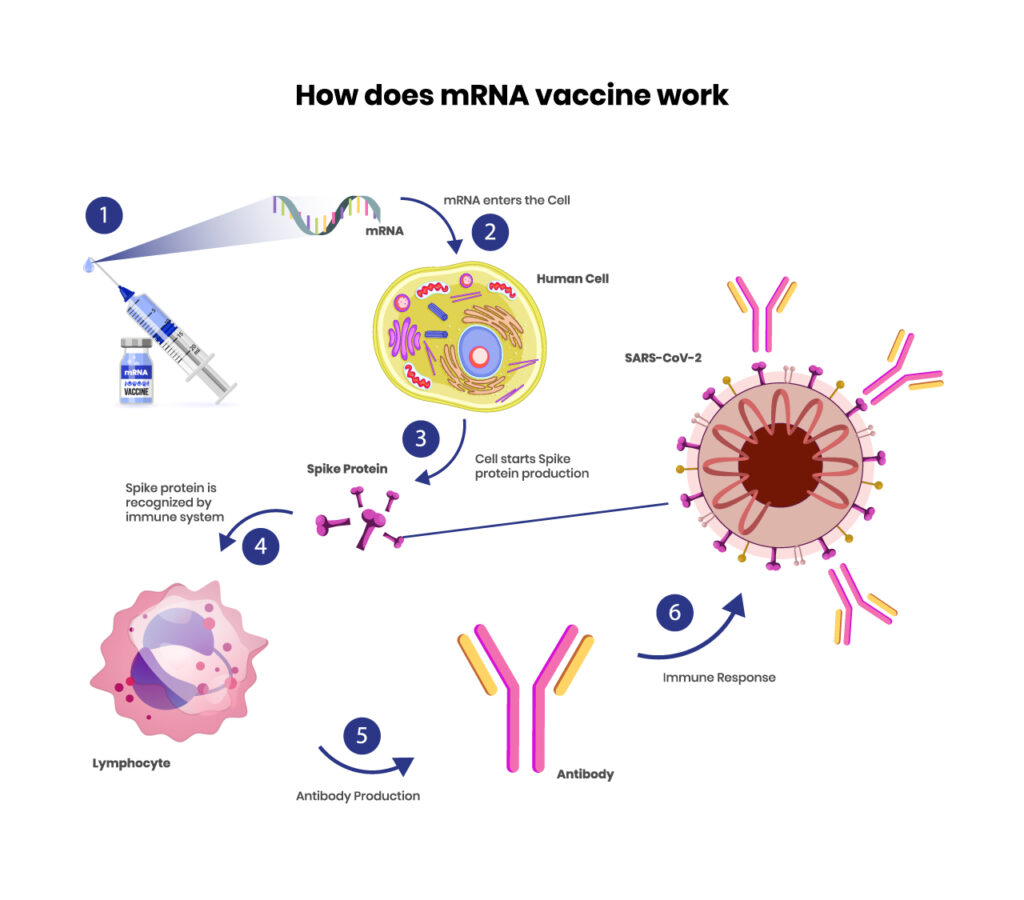
If you go back to your basic biology lessons, you will remember that our genetic code is formed by DNA which is made up of four nucleotide bases, A, G, C, and T. DNA stays in the nucleus of each cell and the tiny pores of the nucleus serve as gatekeepers and do not let in anything that should not enter it. Pieces of information in the DNA are transcribed into mRNA (A, G, C, and U), then the mRNA is transported out of the nucleus and enters the cytoplasm of the cell. Once there, mRNA is translated by the ribosome and specific proteins are made from amino acids, based upon the instructions carried by that particular piece of mRNA.
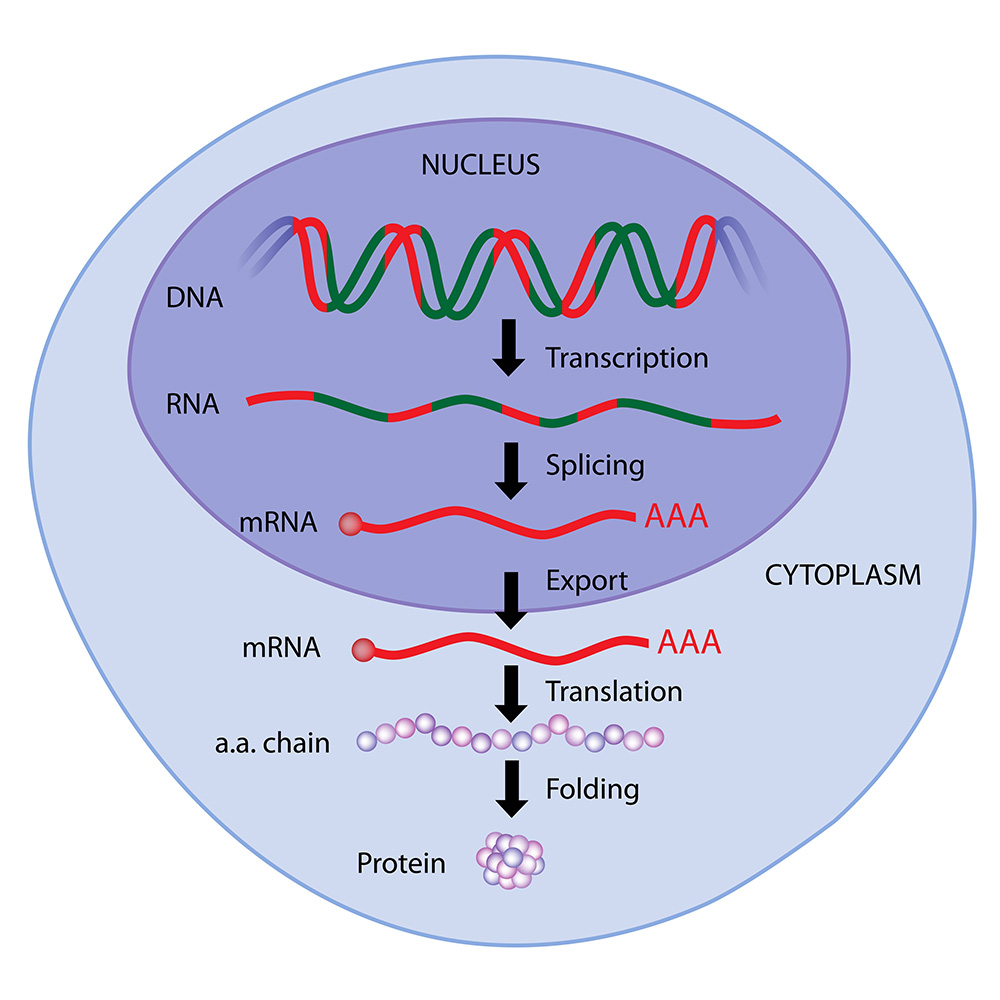
If you look at the image, you can see where mRNA in the cytoplasm is translated into an amino acid chain, creating a protein. mRNA vaccines provide the mRNA, then the body’s natural process of creating proteins takes over. An mRNA vaccine is created with great precision, to carry a synthetic piece of mRNA into the cytoplasm, where it is translated by the ribosome to create the spike protein found on SARS-CoV-2. This mRNA cannot create any other protein than the spike protein that it is coded for, and there is nothing in the vaccination that could transport the mRNA into the nucleus.
mRNA is easily degraded, and after a short amount of time, it is broken down and can no longer be used to create protein. Additionally, human cells do not last for long. So, any cell containing the mRNA will also die in a matter of weeks or months.
Are the mRNA COVID-19 vaccines exposing you to the virus?
No. They are tiny, synthetic pieces of RNA, that only code for one protein on the virus. mRNA vaccines do not contain the virus in any form, or any infectious element, so you are not exposed to the virus through the vaccine at all. The spike protein that they instruct your cells to produce is completely harmless on its own (2).
This means there is an added benefit that, unlike with some other types of vaccinations, there are no concerns about virus shedding or exposing other people for a short time after you have been vaccinated.
If the vaccine isn’t exposing us to the virus, then why are people having reactions?
Most reactions are simply the immune system responding as it learns to recognize and destroy the spike protein. Although you are not exposed to the actual virus, as your immune system mounts a response against the spike protein, it may still respond with localized swelling and pain, inflammation, fever, headache, fatigue, and joint or muscle aches (3). This is a common response with any vaccination and most people will not experience these symptoms or will have a very mild response.
So why get the vaccine if it can cause an immune response?
After your body creates the spike protein, your immune system registers it as an intruder and creates antibodies so it can mobilize quickly when encountering the actual virus. This is important because it normally takes the human body 2-3 weeks to create antibodies when it encounters a virus for the first time, during which, you can become very ill. However, when your body can recognize a pathogen and respond right away, it destroys the virus before it has time to replicate enough to cause you to become ill.
What about the reports of allergic reactions? Are any of the ingredients dangerous?
Like any other vaccine, medication, and even food, most people will tolerate mRNA vaccinations just fine, but a small percentage will have an allergic reaction. mRNA vaccines do also have an advantage over some other types of vaccines in this arena because they do not contain common allergens such as egg or any of the preservatives used in other vaccines (4). They also do not require toxic chemicals or cell cultures during the manufacturing process, avoiding any associated risks (1), nor were they created with fetal cell or animal lines. Additionally, the vial stoppers are not made with latex. If you are concerned about the ingredients, you can see the complete list of ingredients for the Moderna vaccine here and the Pfizer-BioNTech vaccine here.
So far, only people who are allergic to polyethylene glycol or polysorbate, or who have had any prior reaction (even a mild reaction) to these vaccinations are being warned to not get the vaccine. People who have a history of anaphylaxis should discuss it with their doctor and take precautions if they decide to get the vaccine.
Although monitoring is still ongoing, the number of people who reported a severe reaction after the Moderna or Pfizer-BioNTech COVID-19 vaccine is still a far lower number than those who become severely ill from the virus itself. (A severe reaction includes allergic reactions and non-allergic adverse events.)
According to the CDC, as of February 11, 2021, there have been a total of 27,127,858 cases of COVID-19 in the U.S., and 470,110 deaths. It is estimated that approximately 10-15% of people with COVID-19 become severely ill.
In contrast, on January 10, the first dose of the Moderna COVID-19 vaccine had been administered to 4,041,396 people in the United States, and 1,266 (0.031%) adverse events were reported with only 108 being classified as severe.
On January 20, an additional 1,893,360 people had received the first dose of the Pfizer-BioNTech vaccine and 4,393 of them reported an adverse event (.23%), and only 175 of these were classified as severe.
You can see that becoming ill with COVID is far more dangerous than getting an mRNA vaccine. Additionally, if you contrast the rate of adverse events to that of other vaccinations, these rates are comparable and lower than many. Of course, we will need to see if these rates remain the same after the second dose, but these numbers are similar to those seen in the clinical trials.
*** UPDATED INFORMATION on October 8, 2021:
As of September 22, over 223 million doses of the Pfizer BioNTech vaccines and over 151 million doses of the Moderna vaccine have been administered in the United States according to Statista. This means that nearly 375 million doses of mRNA Covid vaccines have now been administered in the United States.
As you can imagine, many different entities are tracking the vaccine for effectiveness in the real-world situation, as well as to monitor adverse events. Only two new serious adverse events have revealed themselves: myocarditis and pericarditis which are forms of heart inflammation. 1,541 cases have been reported in the United States, with 892 of these confirmed. The FDA updated the information on both the Pfizer/BioNTech and Moderna Covid-19 vaccines to reflect that there is increased risk of these conditions. However, even if all 1,541 cases are attributed to the vaccine, when you compare it to the 375,000,000 doses administered, that is still a very low risk of just over four people for every million doses. Especially considering that 1 in 500 people in the United States have now died of Covid-19. (That is equivalent to 2,000 deaths per million people – and that doesn’t include how many people have become severely ill from Covid.)
There have been 5,730 deaths reported in the U.S. that are being investigated as to whether they are related to an mRNA vaccine. Even if all reported deaths are found to be related to the vaccination, this is only .0015%. That is a much better rate than 1 in 500 (.2%) of people who have died.
*A note about adverse events. ALL vaccines carry varying degrees of risk for adverse events – including common childhood vaccines that most children receive by the age of 10. This is not new to Covid vaccines. We have just never had a worldwide vaccination of billions of people in such a short timeframe before this. So, while it may seem like the risk of serious adverse events is exceptionally high, a serious reaction in just a few people per million who are vaccinated is actually quite small – especially when compared to the risks associated with the disease. Everyone involved in deciding whether to advance any vaccine through each stage of development and approval has intensively weighed the risk of the vaccine versus the risk of the disease.
Isn’t the technology too new to be trusted? If mRNA vaccines really work and can be produced so quickly, then why haven’t any been used before?
Technically, mRNA vaccines are not new. They, along with other RNA therapeutics, have been in development for decades, and many mRNA vaccines were in phase 1, 2, and 3 trials before the COVID-19 vaccines were created. There simply has not been the level of urgency to produce them to combat a virus as we have seen in this pandemic. The arrival of SARS-CoV-2 highlighted the critical need for solutions, and the development of mRNA vaccines along with other oligonucleotide therapies became an urgent priority. (Read more about oligonucleotide therapeutics in the fight against COVID-19 here.)
The potential of mRNA vaccines was restricted initially because mRNA is unstable, delivery is difficult, and they often created an undesirably strong immune response (1, 5). mRNA by itself is easily detected by the immune system, and it needs to avoid detection and be delivered into the cell (4). Each vaccine requires determining what portions of mRNA will create the desired response and what formulations will create an effective immune response without creating too strong an immune reaction (1).
Once these, and a myriad of other problems were solved, pharmaceutical companies were not eager to invest in mRNA vaccines for infectious diseases because there are many other effective vaccine platforms in use.
Moderna is one exception, and they have focused on developing and improving mRNA vaccines for infectious diseases for a decade. They have completed two phase 1 studies for influenza strains that were found to be both safe and effective (6). Another phase 1 trial for a Zika virus vaccine is underway, as is a phase 2 dose-finding trial for a cytomegalovirus vaccine. Undoubtedly, this contributed to their ability to respond so quickly during this pandemic and allowed them to produce one of the first vaccines to receive emergency authorization. So, while it is true that we do not have long term data on these specific vaccines, we do have data on vaccines with similar platforms and delivery methods that show longer-term safety.
Instead, researchers have focused on utilizing mRNA vaccines in another arena that is currently lacking any form of disease prevention, cancer (1, 4, 7). Think about that for a minute. mRNA vaccines have the potential to destroy cancer. This is not just an idea they are working with. It is a reality for the near future. There are multiple cancer vaccines currently in phase 2 and 3 trials.
Beyond development, manufacturing and distribution are other aspects that have needed to be considered and fine-tuned. One example of an area that still needs improvement is the extremely low temperatures that mRNA vaccines need to be stored at to remain stable. As with any product, manufacturers want their product to be as user friendly as possible, and maintaining a cold chain is not ideal. With more time, this would no longer be a concern. However, due to the urgency created by COVID-19, pharmaceutical companies decided to go ahead with the manufacturing capabilities they currently have.
A final area that needed to be addressed was determining the proper classification and guiding principles to gain approval by the FDA, EMA, EU, and other regulatory agencies. Many of these considerations have been resolved and, once the rest have been corrected and fine-tuned, mRNA vaccines will be an incredible tool in the fight against cancer and will be able to swiftly provide solutions to meet future pandemics. As they become more widely used, manufacturing capabilities will be greatly expanded, and development and manufacturing processes will be further streamlined and may be able to create and distribute a vaccine far more swiftly than the amazing 1 year it took during this pandemic.
How do we know that it is really safe if the development and approval processes were so rushed?
As discussed in the last point, the development process has been underway for decades. Moderna and Pfizer-BioNTech did not start from the beginning and produce vaccines in less than a year. They used the platforms they had spent years developing and perfecting, then inserted the necessary piece of genetic code and completed the design for an effective mRNA vaccine.
You can think about it this way. Once computers, operating platforms, and software were created, it was a much simpler matter to provide software updates to make small changes. However, we didn’t go from the first computers to today’s smartphones and easily installed apps and updates in just a year or two. The decades of development have brought us to the place where software is easily created and incorporated into existing platforms.
mRNA vaccines operate under a somewhat similar process. Since producing mRNA vaccines uses a process of biochemical synthesis, once the genetic code of a virus is known, the piece of mRNA needed to create an immune response can quickly be synthesized and incorporated into the existing platform to create an effective vaccine.
Approval processes were not changed for these vaccines. You can view details about the FDA’s process here and the European Union’s process here. All new vaccinations and medications go through rigorous testing, and the final steps include 3 phases of human trials. The final phase requires testing thousands of people. Moderna’s phase 3 trial included 30,420 participants, of which half received the vaccine (8), and Pfizer-BioNTech’s phase 3 trial included 43,548 participants, with just under half receiving the vaccine (9). The other half in both groups received a placebo in order to accurately compare results between the participants who received a vaccine or a placebo. Both vaccines met the requirements of safety and efficacy, with an astonishing 94.1% and 95% rate of being effective in preventing COVID-19 illness (8, 9).
Because of the global threat, the FDA and other regulatory agencies around the world allowed Emergency Use Authorization, once there was scientific evidence that the mRNA vaccines would work. They still required that all three phases of human trials provide clear proof that the vaccines met strict standards of safety and effectiveness. The regulatory agencies have also given COVID-related vaccines and medications priority (moving them to the front of the line) and sped up their own internal review process so it takes weeks rather than the 4 – 6 months that it normally would.
The vaccines will continue to be monitored for safety and efficacy, and if either of these appears to be an issue the vaccine would lose its Emergency Use Authorization. They will also be monitored to determine safety and effectiveness in a wider range of patient populations, such as pregnant women, children, and immune-compromised people, and also to determine if it remains effective against new variants of SARS-CoV-2.
*** UPDATED INFORMATION on October 8, 2021:
The Pfizer-BioNTech COVID-19 Vaccine provided evidence that the vaccine was both safe and effective for people 16 years and older, and received full approval from the U.S. FDA on August 23, 2021, for two doses. Emergency Use Authorization had previously been expanded on May 10, 2021, to allow vaccines for individuals 12 through 15 years of age and was expanded again on September 22, 2021, to include a booster dose for those 65 years and older, or those who are at high risk of severe COVID-19.
Moderna has also provided updated evidence and requested FDA approval of a booster that is half of the original dose. It is possible that the proposed lower dose will reduce the rates of adverse events even further.
Many other regulatory agencies around the world have approved both mRNA vaccines for children 12-17, and boosters for various high-risk groups, based on current data that shows it is both safe and effective (11, 12).
Manufacturers also did their part to speed up the process. While in one phase, if a trial began indicating that the vaccines were both safe and effective, manufacturers began producing enough vaccines to begin the next phase. During phase 3, they gambled that they would receive emergency authorization and/or approval and began manufacturing the vaccine so that doses would be ready to distribute immediately upon authorization. Since mRNA vaccine production is laboratory-based and comparatively fast and simple, this allowed the companies to deliver far greater numbers of doses much more quickly than would be possible with traditional vaccines.
Together, all this information shows that it was not the scientific process, clinical trials, and safety standards that were relaxed and sped up. Rather, the red-tape, lengthy wait times for regulatory agencies to review the data, and manufacturing processes that were sped up to bring effective vaccines to the world, after they had been perfected over decades.
*** UPDATED INFORMATION on October 8, 2021:
Can you still get COVID after receiving the vaccines?
As you’ve probably heard, the short answer is yes, and it is being referred to as breakthrough Covid. However, the chance of becoming ill is very low and if you develop symptoms they are most likely going to be mild. Here is an explanation to help you understand why you might experience breakthrough Covid.
Vaccines are not a magic shield that prevents an illness from entering your body. A basic understanding of how vaccines and the immune system work and the role viral load plays in illness will help this make sense.
The immune system has two main parts – the innate immune response and the adaptive (or acquired) immune response. The innate immune system immediately goes to work to kill any foreign pathogen. The adaptive immune system takes longer to become active in fighting the pathogen, as it has to learn to identify the intruder, then create a specialized response to destroy it. It is especially useful when the virus or bacteria replicates so quickly that the innate immune response cannot completely destroy it and you start experiencing symptoms of illness because, when the adaptive immune response is fully involved, you now have both systems working to attack the disease. The adaptive immune system is responsible for creating antibodies that remember a pathogen so it can kick in and fight off the disease faster when you are exposed in the future, and it is also why we can become fully immune to some diseases.
Vaccines take advantage of your body’s natural defenses to help combat disease. They introduce an antigen for a specific pathogen to the body, which helps it create antibodies to the disease. In the case of Sars-CoV-2, the antigen used in the mRNA vaccines is the spike protein. The first time the body is exposed it can take around two weeks to create enough antibodies to fight the disease. However, after the first exposure, the body will remember that antigen and respond much faster and stronger than it is capable of doing the first time. As we have seen with Sars-CoV-2, it can replicate so quickly that a person is likely to fall seriously ill before the immune system can get ahead of it, which is why so much time, effort, and money was poured into creating vaccines. Many times, a vaccine is given over 2-3 doses to create an even stronger response, so the body can fight the virus better than it could with just one dose. After the adaptive immune system is trained to recognize Sars-CoV-2, people have a much higher likelihood of fighting it off before displaying any symptoms at all and sometimes even before there is enough of the virus to be detected by testing. Or, if they do develop symptoms, it generally presents as a mild form of the illness.
Although the adaptive immune system responds much faster and more effectively after it has already been exposed to a virus, it does still take some days to respond and eliminate the threat. How long it takes and how well it works is directly tied to something called the viral load.
Viral load simply refers to how much of the pathogen is in your body.
Once a virus enters your bloodstream it will quickly infect a cell and that cell will be programmed to produce many other viruses which go on to infect other cells until there is so much of it in your body that your immune system can no longer completely fight it off and you develop symptoms. So, the healthier you are the faster your immune system can fight off the virus and prevent it from reproducing as quickly. It is also important to note that it could also take much longer for your immune system to fight off the disease, even after being fully vaccinated, if your immune system is weak (from a preexisting condition, poor diet, lack of sleep, high levels of stress, anxiety, nutrient deficiencies, lack of exercise, or other factors).
Additionally, when you have previously been exposed, your adaptive immune system and innate immune system can start working immediately to eliminate the virus. This means that the virus has a much shorter time to replicate, and you are far less likely to develop symptoms because your viral load never gets high enough.
The other side of the coin is how much of the virus you are exposed to in the first place. Whenever an infected person sneezes, coughs, or even breathes (depending on the virus) the infectious form of the virus is transmitted and other people can become ill. When higher concentrations of a pathogen enter your body there is a higher likelihood that some will get past your immune system, infect cells, and start reproducing.
This is why you are much less likely to develop symptoms if someone in the grocery store sneezes behind you than if your ill child sleeps in your bed, breathing in your face and coughing on you all night long. It is also the reason why medical personnel are at a much higher risk of becoming severely ill. They are being exposed to high concentrations all throughout the day, for days on end.
So, even after vaccination, it does still take a few days or longer for your body to completely eliminate the threat. This is why you can still test positive for Covid-19 and even display symptoms, especially if you have high exposure to the virus.
Can you really transmit COVID after being fully vaccinated against it?
As we saw in the previous question, you can still test positive and even develop symptoms after a vaccination, although the chance of developing moderate to severe illness is much, much lower. Any time you have enough of Sars-CoV-2 that you test positive or develop symptoms, you can also transmit the virus.
How long are the vaccines effective? Do we really need a booster?
Data is still being collected, both in clinical trials and through real-world data (13, 14, 15). Both Pfizer/BioNTech and Moderna have released results after monitoring individuals 4 – 6 months after vaccination showing that while there is still excellent protection, effectiveness is slowly waning. Real-world data supports this. One report that included U.S. adults without immunocompromising conditions stated that from March 11–August 15, 2021 vaccine effectiveness against COVID-19 hospitalization was 93% for the Moderna vaccine and 88% for the Pfizer-BioNTech vaccine (16).
Moderna has released recent results from its Phase 3 trial which found that the two-dose vaccine was still 93.2% effective in preventing Covid-19 illness and 98.2% effective at preventing severe disease when measured five months after being fully vaccinated (13). A Kaiser study found that it was 87% effective against COVID-19 diagnosis and 96% effective against COVID-19 hospitalization.
Pfizer-BioNTech’s two-dose vaccine was 91% effective up to six months after receiving the second dose, and 97% effective at preventing severe disease (14).
An interesting report from Israel showed that protection provided by both doses of the Pfizer-BioNTech vaccine had decreased to 85% at 6 months after the second dose. After receiving the booster dose, efficacy was restored to original levels (17).
While the mRNA vaccines still display greater effectiveness against SARS-CoV-2 infection after 6 months than the other vaccine types, booster doses are still being considered. A booster provides higher levels of protection for a longer period of time, and this will help minimize the number of people hospitalized. It will also confer greater protection as you are exposed to new variants that may replicate faster than the original variant.
Another reason to consider a booster is that the more a virus circulates, the greater chance of a higher number of variants surfacing.
Fortunately, it does seem that both vaccines provide high levels of protection against the variants that have emerged. Although it may not be quite as effective against some variants, it still provides excellent protection for most (18, 19, 20, 21). While still being monitored, it may not be necessary to provide regular boosters to protect against specific variants as many originally feared. Moderna stated they are still investigating four vaccine candidates in clinical trials to address specific variants, but that the original vaccine is still highly effective against Delta.
What is the difference between mRNA Covid vaccines and other types of Covid vaccines?
Pfizer/BioNTech and Moderna developed the mRNA vaccines that we have been discussing in this article. In addition to the two mRNA vaccines, four others are currently approved by WHO and used in many countries worldwide. These are the Janssen/Johnson & Johnson vaccine, Vaxzevria by AstraZeneca-Oxford, CoronaVac by Sinovac, and BBIBP-CorV by Sinopharm. The other four Covid vaccines currently recommended by WHO are either viral vector vaccines or inactivated vaccines. (See this review for a detailed overview of the main types of SARS-CoV-2 vaccines.)
Inactivated Covid vaccines use an inactivated version of the SARS-CoV-2 virus. The inactivated virus cannot replicate and is mixed with an adjuvant to stimulate the immune system. After it is administered, the immune system learns to recognize the virus and creates antibodies to respond to the virus. This is the same type of vaccine that is approved for rabies and hepatitis A.
Viral vector vaccines are composed of a weakened version of a harmless adenovirus that has been modified to contain genetic material of SARS-CoV-2 spike protein. Your cells make copies of the spike protein so your immune system learns to recognize it and can quickly recognize and fight off SARS-CoV-2.
All three types of vaccines ultimately train your body to recognize the virus and produce antibodies so your body can fight off SARS-CoV-2 much more quickly and effectively when you are exposed to the virus. They do have different mechanisms of action, different rates of efficacy, different dose requirements, and different adverse events. It is important to note that efficacy is an overall number, and it may be less effective for certain populations, such as those over 65 years of age. Here is a brief summary of each vaccine.
Efficacy: 95% effective at preventing laboratory-confirmed infection, 87.5% for severe disease
Type: lipid nanoparticle with mRNA vaccine
Doses: 2 doses, 21 days apart (an additional booster dose for certain individuals)
Serious adverse events: Rare allergies and anaphylaxis, rare cases of myocarditis and pericarditis
Efficacy: 94.1% effective at preventing laboratory-confirmed COVID-19 infection, 100% for severe disease
Type: lipid nanoparticle with mRNA vaccine
Doses: 2 doses, 28 days apart
Serious adverse events: Rare temporary facial paralysis (Bell’s Palsy), rare cases of myocarditis and pericarditis
Efficacy: 66.3% effective at preventing laboratory-confirmed COVID-19 infection (72% in the US), 85.4% against severe disease, and 93.1 % against hospitalization
Type: Viral Vector (non-replicating)
Doses: 1 dose (However, recent research shows that a booster after six months may greatly increase efficacy. J&J has indicated that they will be seeking FDA approval for a booster.)
Serious adverse events: Rare cases of blood clots, thrombocytopenia, Guillain-Barré Syndrome
Vaxzevria by AstraZeneca-Oxford
Efficacy: 63.09% effective against symptomatic SARS-CoV-2 infection, 100% against severe disease
Type: Viral Vector (non-replicating)
Doses: 2 doses, 8-12 weeks apart
Serious adverse events: Rare thromboembolic events, rare cases of blood clots, pulmonary embolism, thrombocytopenia
Efficacy: Multiple trials produced varying but similar results with one of the largest reporting: 66% effective against symptomatic COVID-19, 88% against hospitalization, 90% against ICU admissions, and 86% against deaths
Type: Inactivated SARS-CoV-2
Doses: 2 doses, 14-28 days apart
Serious adverse events: None reported
Efficacy: 79% effective against symptomatic COVID-19 and hospitalization
Type: Inactivated SARS-CoV-2
Doses: 2 doses, 21 days apart
Serious adverse events: Rare serious nausea, blood clot, and acute disseminated encephalomyelitis (a rare neurological disorder)
Other vaccines are being used around the world, but as they are not currently approved by WHO or widely approved for use in multiple countries.
Can people who have been vaccinated “shed” and cause others to experience health effects?
Not at all. This idea arises from the fact that vaccines with a live weakened version of a virus can shed. However, mRNA vaccines do not contain any part of the actual SARS-CoV-2 virus at all.
If you remember from the question above “Why are these called genetic vaccines? Does this mean the vaccine can change your DNA?” the vaccine simply carries a bit of genetic code that tells your cells to form a spike protein. That spike protein is the same as the spike proteins on SARS-CoV-2, so your body can recognize and fight SARS-CoV-2 more quickly and effectively when encountered in the future. (It may be helpful to note that all three main types of Covid vaccines utilize the spike protein in different ways and none of them contain the live virus.)
mRNA vaccines do not, and in fact cannot, replicate to create new cells that you can pass on to others. The protein is created as a new part of normal cells in your body, which the body attacks and destroys, are not infectious in any way, and which you are not transmitting to others. Additionally, the mRNA in the vaccine is fragile (which is why it needs to be stored at such low temperatures) and is very easily degraded by enzymes in your body. Once in the body, it lasts just long enough to do its job before it is completely destroyed within a day or two. Therefore, it is completely impossible for vaccine shedding from an mRNA vaccine.
Can Covid-19 mRNA vaccines cause infertility in women or men?
When using the genetic code of the spike protein to create vaccines was first being discussed, some people heard “spike protein” and associated it with another, completely different spike protein called syncytin-1, which is involved in the growth and attachment of the placenta during pregnancy. It was theorized that the vaccine could make the woman’s body fight this other spike protein and affect fertility. From here, it went off into general theories about infertility in general – with no basis in the way human biology works.
Luckily, our bodies are more intricate than that. Each spike protein is like a very specific key that fits into a specifically shaped lock on the antibody. Unless they are an exact match, an antibody will not recognize and initiate an immune response against the spike protein.
To further prove that the vaccines do not affect fertility, a study showed pregnancy outcomes of women who received an mRNA Covid vaccine in the United States. “Among 3958 participants enrolled in the v-safe pregnancy registry, 827 had a completed pregnancy, of which 115 (13.9%) were pregnancy losses and 712 (86.1%) were live births (mostly among participants vaccinated in the third trimester) (22).” While that may sound like a high number of miscarriages, 10-20% of known pregnancies in the US currently end in miscarriages (23) which makes 13.9% completely normal (although devastating to the woman experiencing it). Another study in Israel on women who delivered from January through June of 2021 found that receiving the mRNA Covid vaccine in the second or third trimester had “no adverse effects on pregnancy course and outcomes” (24).
It is also interesting to note that mothers who breastfeed pass their antibodies on to the baby. Antibodies to SARS-CoV-2 are no exception, and antibodies produced after receiving the Covid vaccine or being infected with the virus have also been found in breastmilk (25, 26). A study of 84 women in Israel showed that breast milk contained anti–SARS-CoV-2-specific IgA and IgG antibodies after vaccination. By week 5 (two weeks after the second dose) 97% of breast milk samples tested positive for IgG (25).
In Conclusion:
In March of 2019, Cuiling Zhang et al. predicted that “mRNA-based vaccines can fill the gap between emerging pandemic infectious disease and a bountiful supply of effective vaccines” (4). Less than two years later this prediction has proven to be true. mRNA vaccines were two of the first to meet the dire need of a vaccine to slow the global health crisis caused by SARS-CoV-2. As improvements are made in the development, manufacturing, and distribution of mRNA vaccines, they will become even more perfectly suited to prevent future pandemics.
As mentioned earlier, mRNA has many more therapeutic uses beyond infectious disease vaccines. Click here to read more.
References:
- Pardi, N., Hogan, M., Porter, F. et al.mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov 17, 261–279 (2018). doi.org/10.1038/nrd.2017.243
- “Be Unique, Treat Individualized.” BioNTech, biontech.de/covid-19-portal/mrna-vaccines.
- “Get the Facts about COVID-19 Vaccines.” Mayo Clinic, Mayo Foundation for Medical Education and Research, 3 Feb. 2021, https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-vaccine/art-20484859
- Zhang, C., Maruggi, G., Shan, H., & Li, J. (2019). Advances in mRNA Vaccines for Infectious Diseases. Frontiers in immunology, 10, 594. doi.org/10.3389/fimmu.2019.00594
- Komaroff, Anthony. “Why Are MRNA Vaccines so Exciting?” Harvard Health Blog, Harvard Health Publishing, 19 Dec. 2020, www.health.harvard.edu/blog/why-are-mrna-vaccines-so-exciting-2020121021599.
- Feldman, R. A., Fuhr, R., Smolenov, I., Mick Ribeiro, A., Panther, L., Watson, M., Senn, J. J., Smith, M., Almarsson, Ӧ., Pujar, H. S., Laska, M. E., Thompson, J., Zaks, T., & Ciaramella, G. (2019). mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine, 37(25), 3326–3334. //doi.org/10.1016/j.vaccine.2019.04.074
- Fiedler K, Lazzaro S, Lutz J, Rauch S, Heidenreich R. mRNA Cancer Vaccines. Recent Results Cancer Res. (2016) 209:61–85. doi: 10.1007/978-3-319-42934-2_5
- Baden, L. R., El Sahly, H. M., Essink, B., Kotloff, K., Frey, S., Novak, R., Diemert, D., Spector, S. A., Rouphael, N., Creech, C. B., McGettigan, J., Khetan, S., Segall, N., Solis, J., Brosz, A., Fierro, C., Schwartz, H., Neuzil, K., Corey, L., Gilbert, P., … COVE Study Group (2021). Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. The New England journal of medicine, 384(5), 403–416. doi.org/10.1056/NEJMoa2035389
- Polack, F. P., Thomas, S. J., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., Perez, J. L., Pérez Marc, G., Moreira, E. D., Zerbini, C., Bailey, R., Swanson, K. A., Roychoudhury, S., Koury, K., Li, P., Kalina, W. V., Cooper, D., Frenck, R. W., Jr, Hammitt, L. L., Türeci, Ö., … C4591001 Clinical Trial Group (2020). Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. The New England journal of medicine, 383(27), 2603–2615. doi.org/10.1056/NEJMoa2034577
- “Understanding MRNA COVID-19 Vaccines.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 18 Dec. 2020, www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/mrna.html.
- Frenck, R. W., Jr, Klein, N. P., Kitchin, N., Gurtman, A., Absalon, J., Lockhart, S., Perez, J. L., Walter, E. B., Senders, S., Bailey, R., Swanson, K. A., Ma, H., Xu, X., Koury, K., Kalina, W. V., Cooper, D., Jennings, T., Brandon, D. M., Thomas, S. J., Türeci, Ö., … C4591001 Clinical Trial Group (2021). Safety, Immunogenicity, and Efficacy of the BNT162b2 Covid-19 Vaccine in Adolescents. The New England journal of medicine, 385(3), 239–250. doi.org/10.1056/NEJMoa2107456
- Glatman-Freedman, A., Hershkovitz, Y., Kaufman, Z., Dichtiar, R., Keinan-Boker, L., & Bromberg, M. (2021). Effectiveness of BNT162b2 Vaccine in Adolescents during Outbreak of SARS-CoV-2 Delta Variant Infection, Israel, 2021. Emerging infectious diseases, 27(11), 10.3201/eid2711.211886. Advance online publication. doi.org/10.3201/eid2711.211886
- El Sahly, H. M., Baden, L. R., Essink, B., Doblecki-Lewis, S., Martin, J. M., Anderson, E. J., Campbell, T. B., Clark, J., Jackson, L. A., Fichtenbaum, C. J., Zervos, M., Rankin, B., Eder, F., Feldman, G., Kennelly, C., Han-Conrad, L., Levin, M., Neuzil, K. M., Corey, L., Gilbert, P., … COVE Study Group (2021). Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. The New England journal of medicine, 10.1056/NEJMoa2113017. Advance online publication. doi.org/10.1056/NEJMoa2113017
- Stephen J. Thomas, Edson D. Moreira Jr., Nicholas Kitchin, Judith Absalon, Alejandra Gurtman, Stephen Lockhart, John L. Perez, Gonzalo Pérez Marc, Fernando P. Polack, Cristiano Zerbini, Ruth Bailey, Kena A. Swanson, Xia Xu, Satrajit Roychoudhury, Kenneth Koury, Salim Bouguermouh, Warren V. Kalina, David Cooper, Robert W. Frenck Jr., Laura L. Hammitt, Özlem Türeci, Haylene Nell, Axel Schaefer, Serhat Ünal, Qi Yang, Paul Liberator, Dina B. Tresnan, Susan Mather, Philip R. Dormitzer, Uğur Şahin, William C. Gruber, Kathrin U. Jansen, C4591001 Clinical Trial Group (2021). Six Month Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. medRxiv 2021.07.28.21261159; doi: doi.org/10.1101/2021.07.28.21261159
- Pilishvili, T., Gierke, R., Fleming-Dutra, K. E., Farrar, J. L., Mohr, N. M., Talan, D. A., Krishnadasan, A., Harland, K. K., Smithline, H. A., Hou, P. C., Lee, L. C., Lim, S. C., Moran, G. J., Krebs, E., Steele, M. T., Beiser, D. G., Faine, B., Haran, J. P., Nandi, U., Schrading, W. A., … Vaccine Effectiveness among Healthcare Personnel Study Team (2021). Effectiveness of mRNA Covid-19 Vaccine among U.S. Health Care Personnel. The New England journal of medicine, 10.1056/NEJMoa2106599. Advance online publication. doi.org/10.1056/NEJMoa2106599
- Self, W. H., Tenforde, M. W., Rhoads, J. P., Gaglani, M., Ginde, A. A., Douin, D. J., Olson, S. M., Talbot, H. K., Casey, J. D., Mohr, N. M., Zepeski, A., McNeal, T., Ghamande, S., Gibbs, K. W., Files, D. C., Hager, D. N., Shehu, A., Prekker, M. E., Erickson, H. L., Gong, M. N., … IVY Network (2021). Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions – United States, March-August 2021. Morbidity and mortality weekly report, 70(38), 1337–1343. doi.org/10.15585/mmwr.mm7038e1
- Bar-On, Y. M., Goldberg, Y., Mandel, M., Bodenheimer, O., Freedman, L., Kalkstein, N., Mizrahi, B., Alroy-Preis, S., Ash, N., Milo, R., & Huppert, A. (2021). Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. The New England journal of medicine, NEJMoa2114255. Advance online publication. doi.org/10.1056/NEJMoa2114255
- Rolando Pajon, Yamuna Paila, Bethany Girard, Groves Dixon, Katherine Kacena, Lindsey R Baden, Hana M El Sahly, Brandon Essink, KathleenM Mullane, Ian Frank, Douglas Denham, Edward Kerwin, Xiaoping Zhao, Baoyu Ding, Weiping Deng, Joanne Tomassini, Honghong Zhou, Brett Leav, Florian Schodel (2021). Initial Analysis of Viral Dynamics and Circulating Viral Variants During the mRNA-1273 Phase 3 COVE Trial. medRxiv 2021.09.28.21264252; doi: doi.org/10.1101/2021.09.28.21264252
- Tang, P., Hasan, M. R., Chemaitelly, H., Yassine, H. M., Benslimane, F. M., Khatib, H. A. A., AlMukdad, S., Coyle, P., Ayoub, H. H., Kanaani, Z. A., Kuwari, E. A., Jeremijenko, A., Kaleeckal, A. H., Latif, A. N., Shaik, R. M., Rahim, H. F. A., Nasrallah, G. K., Kuwari, M. G. A., Romaihi, H. E. A., … Abu-Raddad, L. J. (2021). BNT162B2 and mRNA-1273 COVID-19 vaccine effectiveness against the Delta (b.1.617.2) variant in Qatar. medRxiv 2021.08.11.21261885; doi: doi.org/10.1101/2021.08.11.21261885
- Lopez Bernal, J., Andrews, N., Gower, C., Gallagher, E., Simmons, R., Thelwall, S., Stowe, J., Tessier, E., Groves, N., Dabrera, G., Myers, R., Campbell, C., Amirthalingam, G., Edmunds, M., Zambon, M., Brown, K. E., Hopkins, S., Chand, M., & Ramsay, M. (2021). Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. The New England journal of medicine, 385(7), 585–594. doi.org/10.1056/NEJMoa2108891
- Seppälä, E., Veneti, L., Starrfelt, J., Danielsen, A. S., Bragstad, K., Hungnes, O., Taxt, A. M., Watle, S. V., & Meijerink, H. (2021). Vaccine effectiveness against infection with the Delta (B.1.617.2) variant, Norway, April to August 2021. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin, 26(35), 2100793.doi.org/10.2807/1560-7917.ES.2021.26.35.2100793
- Shimabukuro, T. T., Kim, S. Y., Myers, T. R., Moro, P. L., Oduyebo, T., Panagiotakopoulos, L., Marquez, P. L., Olson, C. K., Liu, R., Chang, K. T., Ellington, S. R., Burkel, V. K., Smoots, A. N., Green, C. J., Licata, C., Zhang, B. C., Alimchandani, M., Mba-Jonas, A., Martin, S. W., Gee, J. M., … CDC v-safe COVID-19 Pregnancy Registry Team (2021). Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons. The New England journal of medicine, 384(24), 2273–2282. doi.org/10.1056/NEJMoa2104983
- Cohain, J. S., Buxbaum, R. E., & Mankuta, D. (2017). Spontaneous first trimester miscarriage rates per woman among parous women with 1 or more pregnancies of 24 weeks or more. BMC pregnancy and childbirth, 17(1), 437. doi.org/10.1186/s12884-017-1620-1Wainstock, T., Yoles, I., Sergienko, R., & Sheiner, E. (2021). Prenatal maternal COVID-19 vaccination and pregnancy outcomes. Vaccine, 39(41), 6037–6040. doi.org/10.1016/j.vaccine.2021.09.012
- Mascellino, M. T., Di Timoteo, F., De Angelis, M., & Oliva, A. (2021). Overview of the Main Anti-SARS-CoV-2 Vaccines: Mechanism of Action, Efficacy and Safety. Infection and drug resistance, 14, 3459–3476. doi.org/10.2147/IDR.S315727
- Perl, S. H., Uzan-Yulzari, A., Klainer, H., Asiskovich, L., Youngster, M., Rinott, E., & Youngster, I. (2021). SARS-CoV-2-Specific Antibodies in Breast Milk After COVID-19 Vaccination of Breastfeeding Women. JAMA, 325(19), 2013–2014. doi.org/10.1001/jama.2021.5782
- Pace, R. M., Williams, J. E., Järvinen, K. M., Belfort, M. B., Pace, C., Lackey, K. A., Gogel, A. C., Nguyen-Contant, P., Kanagaiah, P., Fitzgerald, T., Ferri, R., Young, B., Rosen-Carole, C., Diaz, N., Meehan, C. L., Caffé, B., Sangster, M. Y., Topham, D., McGuire, M. A., Seppo, A., … McGuire, M. K. (2021). Characterization of SARS-CoV-2 RNA, Antibodies, and Neutralizing Capacity in Milk Produced by Women with COVID-19. mBio, 12(1), e03192-20. doi.org/10.1128/mBio.03192-20