Treating Genetic Causes of Dravet Syndrome
In September 2014, identical twins Hudson Blake and Grady Lee were born and appeared seemingly perfect. However, when their mom went to return to work when the boys were 12 weeks old, she started to notice they both had an odd shiver. When Hudson ...
Hope of New Treatments for Chronic Liver Disease MASH (NASH)
In the early stages of Metabolic Dysfunction-Associated Steatohepatitis (MASH), many patients are asymptomatic, though some may feel weak or fatigued or have an ache in their upper right abdomen. While lifestyle changes can help patients diagnosed early enough, more advanced stages of the disease ...
RNA Single-base Editing Therapy that Treats Genetic Lung and Liver Disease Entered Clinical Trials
When Peggy's mom was in her forties, she started to have trouble breathing. Although she had never smoked, she had been exposed second-hand earlier in her life. Eventually, her mother ended up at the Mayo Clinic, where she was the 36th patient to be ...
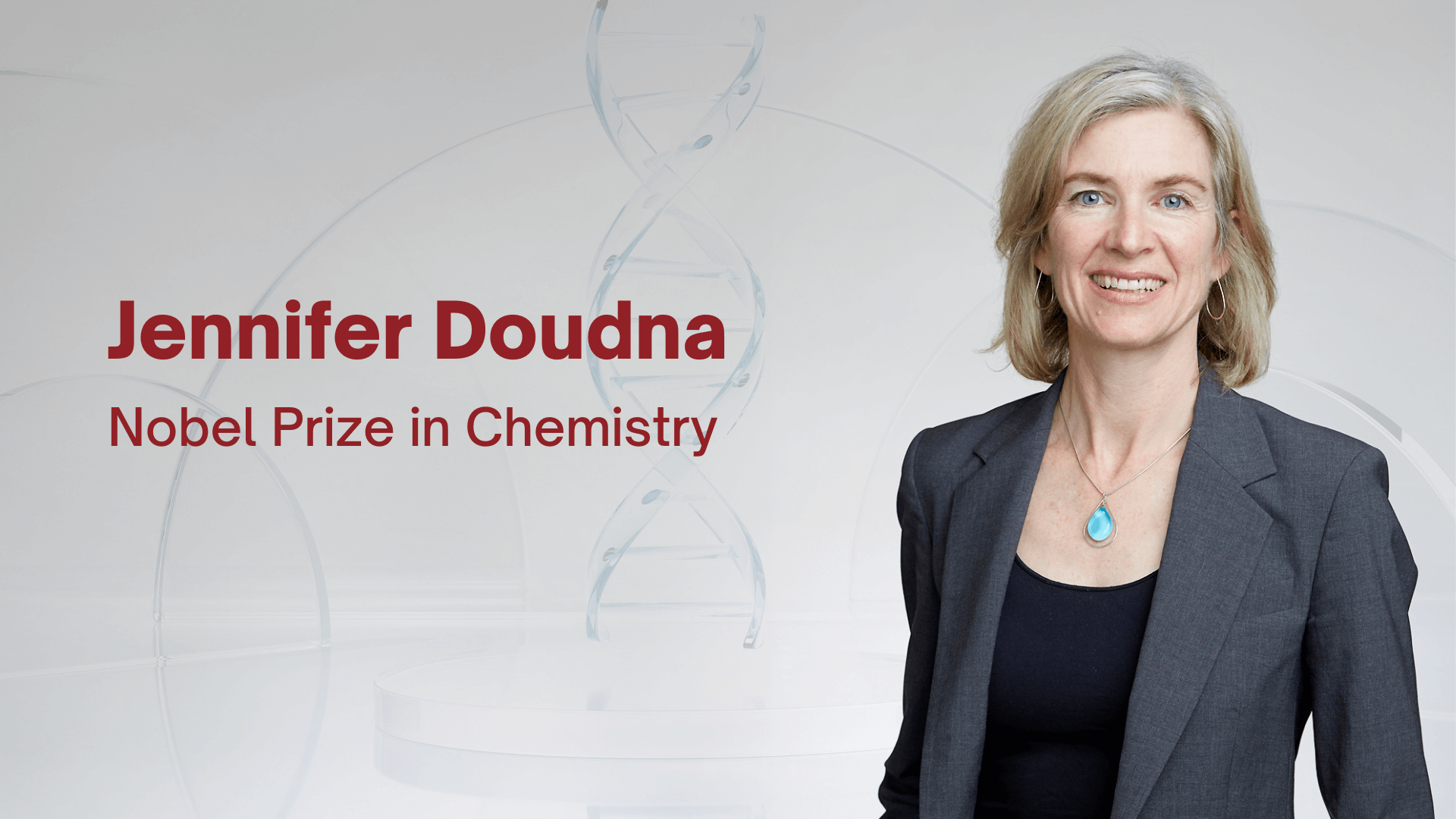
Jennifer Doudna – Seeking to Improve the World with CRISPR
At 10:53 p.m. on Oct. 7, Jennifer Doudna woke to a buzzing sound. Picking up her phone — which she noticed had multiple missed calls and messages — she was greeted by the voice of Nature journalist Heidi Ledford. The day before, Doudna had ...
FDA Approved the First CRISPR Treatment – Potential Cure for People with Sickle Cell Disease
Victoria Gray was diagnosed with sickle cell disease (SCD) when she was just three months old; since then, episodes of pain and frequent hospitalizations have been a part of her life. Many of her dreams seemed like far-off impossibilities when the smallest things, like ...
Follow-up Data Confirms That Inclisiran Provides Long-Term Reduction of LDL-Cholesterol
Cardiovascular disease is the leading cause of death in the United States, exceeding all types of cancer, unintentional injury, and stroke combined. However, updated data from Novartis's open-label trial shows promising long-term results for inclisiran (Leqvio), the first and only small interfering RNA (siRNA) ...
The First FDA Approval for a GalNAc-conjugated ASO
It starts with a pins-and-needle sensation in your feet, or maybe your gastrointestinal tract has become easily irritated, or you've started to lose weight or develop heart problems. Hereditary ATTR amyloidosis can present differently among patients and with symptoms found in far more common ...
Base Editing in Clinical Trials to Treat Acute Lymphoblastic Leukemia
Base editing has experienced a rapid rise in use since it first came on the scene in 2016, with multiple trials underway, testing its ability to treat conditions with precise, single-letter changes to DNA. The technique presents a potentially more accurate and safer method ...
2023 Annual Meeting Highlights
We are pleased to note that this year’s annual Oligonucleotide Therapeutics Society meeting in Barcelona, Spain had the highest involvement of any previous meeting, with 800 in-person attendees, 297 abstract submissions, 260 posters, 47 travel grants, 36 Sponsors, and 29 Exhibitors. We hope all ...
Fanzor: A Programmable RNA-Guided System In Eukaryotes Similar to CRISPR
Researchers have uncovered the first programmable RNA-guided system in eukaryotes that could be even more precise than CRISPR gene-editing. The discovery — led by Feng Zhang at the McGovern Institute for Brain Research at MIT and the Broad Institute of MIT and Harvard — demonstrates ...