Looking for something?
2023 Annual Meeting Highlights

We are pleased to note that this year’s annual Oligonucleotide Therapeutics Society meeting in Barcelona, Spain had the highest involvement of any previous meeting, with 800 in-person attendees, 297 abstract submissions, 260 posters, 47 travel grants, 36 Sponsors, and 29 Exhibitors. We hope all who attended in person or virtually enjoyed their time and will carry the passion and knowledge gained into their future endeavors.
We have curated some highlights from the conference to share. Remember, if you missed something or want to review any of the speakers, sessions, or posters, virtual access is available through the Virtual Meeting Portal until the end of 2023.
There were many great speakers this year, and we thank each person who spoke for sharing their science. Attendees learned about a broad range of many interesting technologies and therapies currently in development, such as a unique endosomal escape enhancer platform, targeting transferrin receptors to transport ASOs over the BBB, prion protein-lowering ASOs, targeting RNA-misprocessing in neurological diseases with CRISPR-Cas systems, reversing DNA methylation abnormalities, delivering siRNA to the skin, and an anti-miR-22 therapy for NAFLD and obesity.
Keynote Presentation

David Liu, PhD, of the Broad Institute at Harvard and co-founder of many companies, including Prime Medicine and Beam Therapeutics, was the Keynote Presenter this year. His phenomenal speech reviewed mammalian cell gene editing technologies, focusing on base editors and prime editors. He pointed out that many therapies currently in development are well-suited for disrupting genes, such as using CRISPR-Cas9 in the treatment of transthyretin amyloidosis. However, they are not well-suited to genetic diseases caused by mutations that result in loss of function, as those types of diseases require precise gene correction to benefit patients.
Base and prime editors can meet this need. Base editors have been developed to directly convert one target base to another without making double-stranded breaks. Six phase 1 clinical trials using base editing therapies are underway. One interesting application of base editing that David Liu discussed was in developing a therapy for treating Spinal Muscular Atrophy (SMA). Frequently in this disease, there is a loss of function mutation in the SMN1 gene which, in humans, creates an imperfect duplication into SMN2. In many patients, a mutation causes exon 7 to be skipped. Base editing allows SMN2 to be targeted to increase SMN protein production, restoring SMN protein levels to wild-type in mice. SMN2 was converted into normal SMN1 in 87% of transduced cells in the CNS and restored motor neuron function levels similar to that of normal mice.
Prime editing, the second gene-editing technology David discussed, was initiated by Andrew Anzalone while he was a postdoctoral fellow in the laboratory of David Liu, PhD. Prime editing uses engineered prime editing guide RNA (pegRNA) that specifies the target site and encodes the desired edit. It does not make double-stranded breaks or use a donor DNA template. David Liu provided an in-depth discussion of the development of prime editing and specific improvements that have been made, including the engineering and evolution of prime editing proteins. For example, the PACE platform was developed to allow evolution campaigns of hundreds or thousands of generations that can take days to a few weeks, rather than hundreds or thousands of weeks. He also explained why there are multiple prime editors, which settings benefit from different types of editors, and provided a simple graphic detailing how to determine when to use each PE6 variant. Finally, potential solutions to maximize the therapeutic reach of gene editing were provided.
2023 Award Winners

This year, OTS once again had the privilege of honoring many outstanding people, including the Lifetime Achievement Award winners from two years: Sudhir Agrawal, D.Phil. and Cy Stein, M.D., PhD. Both men were honored as founders of the field who have demonstrated a long-lasting commitment to the field. At the end of their speeches, the floor was opened to questions, and attendees eagerly asked about the specifics of their work. Dr. Agrawal and Dr. Stein also provided thought-provoking responses to questions such as:
- What direction should young chemists work on?
- Where do you see the field in the next 10-20 years?
- What is the right time and right balance between knowledge and advancing things toward the clinic?
Sudhir Agrawal, D.Phil. was awarded the 2022 Lifetime Achievement Award. He has authored over 300 papers, reviews, and book chapters in the field of antisense research and development and is an inventor on more than 400 patents worldwide. Dr. Agrawal started his work on antisense in the late eighties in the laboratory of Paul Zamecnik, a pioneer of antisense technology. Dr. Agrawal’s research interest has focused on providing drug-like properties to an oligonucleotide sequence, emphasizing the importance of sequence and chemical modifications. His work introduced gapmer antisense design, now widely used in antisense candidates in drugs, both in development and some that have been approved. His work also led to the use of modified RNA for splicing. In his early years of research in antisense development, he addressed the issues of systemic delivery and induction of complement and immune activation.
Dr. Agrawal provided a rich account of his history in the field and key foundational discoveries. Then, after mentioning that he retired in 2017, he went on to describe how he began wondering if he had missed making connections and began a company called Arnay, collaborating with Alloy Therapeutics, to provide the freedom to write different types of structures and connect the dots and see what the experimental data is. Much of his new work is based on the idea of using 3’ and 5’ ends appropriately. To this end, he has developed splitmer antisense and transient cyclic structures, which many in the audience were intrigued by.
Cy Stein, M.D. PhD, was awarded the 2023 Lifetime Achievement Award. A founder of OTS, he was described as an unbiased critic of poor science, because patient safety should be the goal of oligo therapies. His work with oligonucleotides began in March 1987, and he has published extensively on numerous aspects of oligonucleotide biology and biochemistry, focusing on the behavior of phosphorothioate (PS) oligos. Over the years, his laboratory has examined some of the non-sequence specific behaviors of PS oligos, helping to understand in detail the ability of PS oligos to bind to heparin-binding proteins, and how this binding relates to cellular internalization and intracellular localization. Among his numerous other scientific accomplishments, Dr. Stein and his colleagues discovered the ability of PS oligos to enter cells and produce gene silencing in the absence of any carriers (i.e., lipids) or other modifications, called gymnosis.
Dr. Stein’s speech included an in-depth discussion of his early work on phosphorothioate oligonucleotides. During the Q&A, he was asked about what advice he would offer to young investigators, and this was his insightful reply. “Always be true to the science. Go where the science leads you. Never succumb to confirmation bias… Just make sure that you keep an open mind about the results you are seeing. Be alert, be aware of the possibility that there are other possibilities than what you think is happening.” His final advice was, “Maintain your integrity at all costs. Without integrity, you have nothing.”
Julia Vitarello, Mila’s Mom, and the Founder and Chief Executive Officer of Mila’s Miracle Foundation, was awarded the Patient Advocacy Award. Driven by a sense of hope and responsibility, Julia is on a mission to turn the groundbreaking work that went into milasen into a promising platform solution to the drastic problem of rare disease in children. As Tim Yu described when presenting her award, Julia is an extraordinary communicator, strategist, citizen scientist, and community builder with an amazing ability to bridge families, the rare disease community, scientists, policymakers, the general public, and companies, to catalyze conversations about how to make children like Mila seen and heard and how to create approaches to help them.
Alex Felix, PhD, of the University of Pennsylvania Perelman School of Medicine is the recipient of the Next Gen Session: Best Talk Award for his talk titled “miRNA disruption using Steric-Blocking Oligonucleotides as a novel therapeutic strategy for STXBP1-related disorders.” He discussed a therapeutic approach in development to treat STXBP1-related disorders. They are targeting the microRNA binding site, rather than the microRNA directly, to prevent dysregulating many genes and also because by targeting the binding sites, you can potentially block many RNAs that bind to the same site. The SBO lead candidates increased STXBP1 mRNA abundance in patient neurons, specifically rescues miRNA-mediated repression of STXBP1, and the lead has been tested in humanized mice.
Sonia M. Vallabh, PhD and Erik Minkel, PhD of the Vallabh/Minikel Lab at the Broad Institute are the recipients of the Paper of the Year Award – Basic Research Category for their paper titled “A single-cell map of antisense oligonucleotide activity in the brain.” Their work focuses on drug development for prion disease as well as biomarker and regulatory strategy to enable primary prevention trials in pre-symptomatic genetic mutation carriers.
Jinkuk Kim, Ph.D. of KAIST (Korea Advanced Institute of Science and Technology), is the recipient of the Paper of the Year Award – Late Discovery Category for his paper titled “A framework for individualized splice-switching oligonucleotide therapy.” His research has made significant contributions in the fields of oligonucleotide therapy, rare disease, and genomics. Notably, with Timothy W. Yu, he developed milasen, the first fully patient-customized therapy for a rare genetic disease.
Daniel O’Reilly, Ph.D. of the RNA Therapeutics Institute, UMass Chan Medical School, is the recipient of the 2023 Dr. Alan M. Gewirtz Memorial Scholarship – Postdoctoral Fellows and Junior Industrial Professionals. In the Khvorova lab, Dr. O’Reilly is leading Huntington’s Disease research projects, including utilizing siRNA to modulate MMR factors to block somatic expansion. He is also leading a group whose role is to utilize novel chemical scaffolds to enhance RISC loading and cleavage to increase the long-term efficacy of siRNA.
Krystal Johnson, Ph.D. of the UT Southwestern Medical Center, Corey Lab, is the recipient of the 2023 Dr. Alan M. Gewirtz Memorial Scholarship – Graduate Students. Her thesis work expands microRNA’s realm of regulation by exploring disease-relevant conditions where microRNAs and their protein machinery are driven into the nucleus. Dr. Johnson’s research can impact our understanding of intracellular regulation of microRNA action and therapeutic targets that contribute to cancer progression.
Raman Bahal, Ph.D., an associate professor at the University of Connecticut, is the recipient of the 2023 Mary Ann Liebert, Inc. Publishers Young Investigator Award for his outstanding achievements and contributions. Dr. Bahal has published 65 research papers in prestigious journals and his lab is developing new therapeutic modalities for targeting genomic DNA and RNA at the interface of nucleic acid chemistry and nanotechnology.
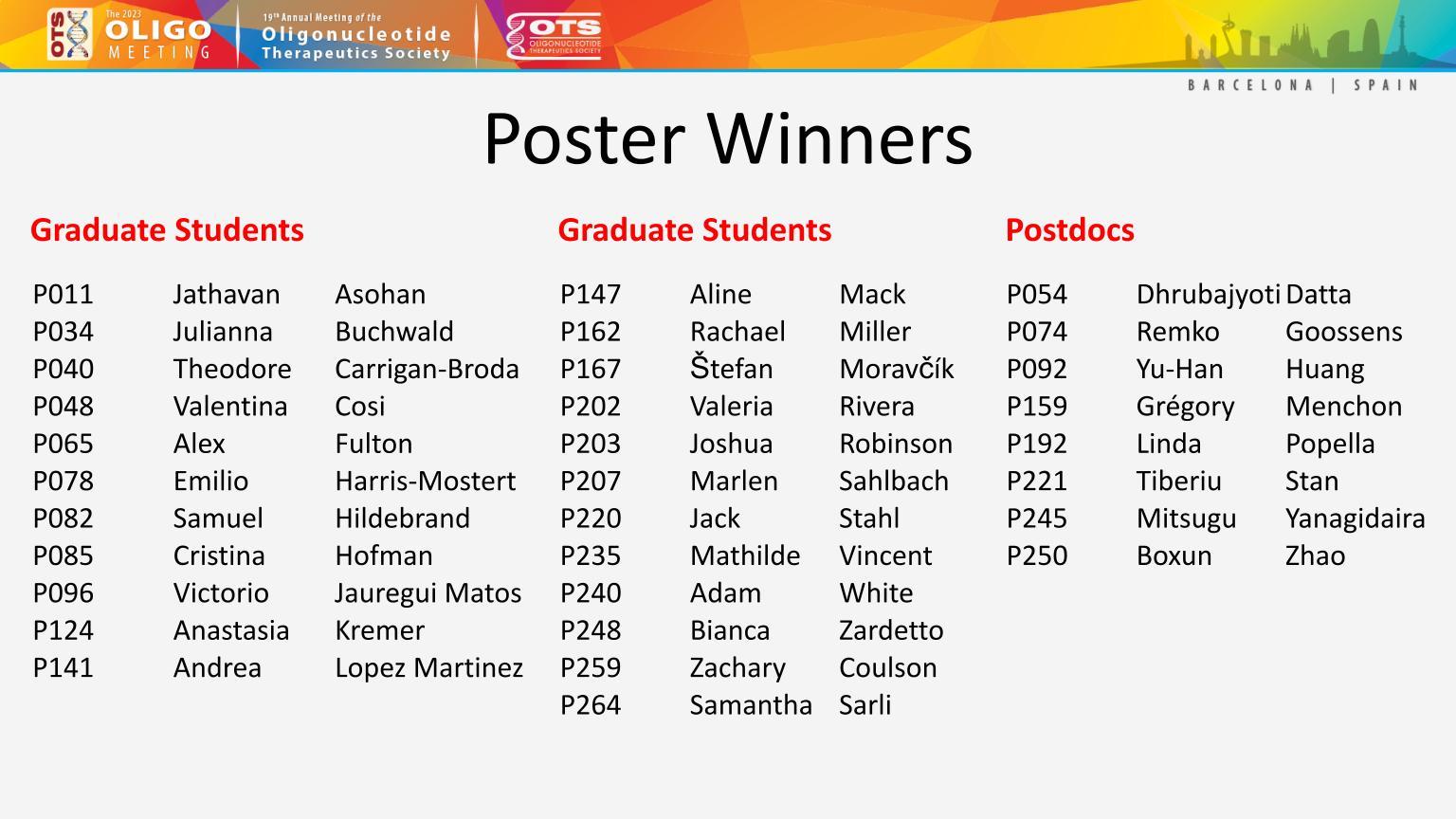
Once again, attendees viewed many excellent Posters this year, and we would like to congratulate this year’s Poster Award Winners.
A Short Overview of the Clinical Session
This year’s clinical session was incredible, as always. First, attendees heard from Jaya Goyal, PhD, of PepGen, as she discussed PGN-EDO51. Designed to treat Duchenne muscular dystrophy (DMD) by targeting exon 51, this therapeutic is a peptide-conjugated oligonucleotide that uses PepGen’s novel Enhanced Delivery Oligonucleotide (EDO) platform. The EDO platform was designed for enhanced uptake and improved tolerability in muscle and other target tissues. Both mouse and NHP models showed promising results. NHPs received three doses via IV, and high levels of exon skipping were displayed in the biceps (77.5%), quadriceps (73.4%), and diaphragm (75.9%), with lower levels (23.7%) displayed in the cardiac left ventricle. Phase 1 healthy volunteer studies of PGN-EDO51 have been completed, in which the drug was generally well tolerated, and it showed a dose-dependent increase in biceps. Connect1-EDO51 is a phase 2, open-label, multiple ascending dose study that has already begun, in which key endpoints are safety biomarkers, dystrophin protein levels, and exon skipping. Connect2-EDO51 is a phase 2, randomized, double-blind, placebo-controlled, multiple ascending dose study planned to be conducted in parallel with Connect1, and data for both studies is anticipated to be shared in 2024.
Mike Flanagan, PhD, from Avidity Biosciences shared data on a Phase 1/2 trial of AOC 1001, which is a monoclonal antibody conjugated with a double-stranded siRNA. AOC 1001 is designed to reduce levels of DMPK RNA in different types of muscle tissue, addressing the root cause of myotonic dystrophy type 1 (DM1). Data shows that AOC 1001 is delivered to the muscle, engages the target (seen through DMPK knockdown), and has an effect on the pathway, which results in early signs of clinical activity. It only takes six months to see improvement in both muscle strength and coordinated functioning of muscles. To demonstrate, Mike Flanagan shared a video of a patient’s visible significant improvement of hand motion from baseline to day 183. The drug was generally well tolerated; however, there was one SAE that resulted in a partial clinical hold which has since been released and the patient is stable. All participants who chose to join the OLE study have stayed in the study. The team is currently identifying the dose and end points for the Phase 3 trial.
Dr. Mark O’Carroll, PhD, discussed an ongoing study of ARO-RAGE, which was sponsored by Arrowhead Pharmaceuticals. RAGE (Receptor for Advanced Glycation End products) regulates airway inflammation in asthma. ARO-RAGE was designed by Arrowhead Pharmaceuticals to reduce production of RAGE in pulmonary epithelial cells as a potential treatment for various muco-obstructive and inflammatory pulmonary diseases such as Asthma. The drug uses Arrowhead’s Targeted RNAi Molecule (TRiM) platform which allows highly specific degradation of targeted mRNA and deep and durable silencing to minimize dose frequency. ARO-RAGE specifically targets pulmonary epithelial cells and is formulated in saline and delivered to the airway by inhalation using a nebulizer. The Phase 1/2a study, which is currently underway, includes a cohort of healthy adults and one of adults with asthma. The key endpoints are safety and target engagement, with a focus on pulmonary toxicity. It has been well tolerated with no serious adverse events. A dose-dependent response has been observed, with a 90% reduction in RAGE at the highest level (184 mg) in healthy adults. Serum RAGE reduction was similar in asthma patients and healthy adults at the 44 mg dose level, and enrollment of asthma patients is still ongoing at the higher doses.
Kirk Brown, PhD, of Alnylam Pharmaceuticals discussed an ongoing phase 1 trial of ALN-APP in patients with early-onset Alzheimer’s disease. ALN-APP reduces production of APP. As APP is the source of all downstream amyloid beta (Aβ) protein species, it is expected that reducing production would also reduce the secretion of Aβ peptides that aggregate into extracellular amyloid deposits and also reduce the intraneuronal products that trigger the formation of neurofibrillary tangles and cause neuronal dysfunction in Alzheimer’s disease. ALN-APP is being evaluated in a Phase 1 study of patients younger than 65 years who have been diagnosed with early-onset Alzheimer’s disease, displaying MCI or mild dementia. Interim data shows that the drug is well tolerated. All adverse events were mild to moderate, and most were consistent with lumber puncture. CSF safety labs and neurofilament light chain do not show any concerning trends. Biomarkers of target engagement, soluble APPα (sAPPα) and soluble APPβ (sAPPβ), both displayed early, sustained, robust reductions at the highest single dose level (75mg). A part B multi-dose study has been initiated and dosing has begun. They have also expanded the CNS platform to other targets and NHP data for these targets was shared.
Patrick Burkett, MD, PhD, of Moderna Therapeutics shared interim data from an ongoing Phase 1/2 study of mRNA-3705, a therapy for Methylmalonic Acidemia (MMA). MMA is a rare disease caused by mutations in several different genes, but most commonly by changes in the MMUT gene resulting in a defective or missing MUT enzyme. The disease usually presents in early infancy and manifests in recurrent episodes of life-threatening metabolic decompensation events (MDEs) and progressive multi-organ damage. mRNA-3705 is an LNP-encapsulated mRNA therapeutic encoding the MUT enzyme to complement the genetic deficiency. It is translated in the cytoplasm and the enzyme is then carried into the mitochondria, where it is functional. The ongoing phase 1/2 study is in the dose optimization stage with a treatment period of 10 doses (one every two weeks) and is evaluating safety and pharmacology. Currently, eleven participants have been dosed with a total of 221 doses administered and the maximum treatment duration is 1.95 years. It has been well tolerated, and all participants who completed the treatment period have opted to enter the long-term extension study. Some mild and moderate AEs have been observed along with one serious AE, which has been resolved and the patient continued on treatment with no reoccurrence. Dose-dependent changes were seen in the two key biomarkers, and promising initial data on clinical endpoints indicate potential decreases in MDE frequency and MMA-related hospitalizations compared to pre-treatment.
Kenneth Newman, MD, from Ionis Pharmaceuticals, discussed donidalorsen, an ASO Therapy that treats patients with Hereditary Angioedema (HAE). HAE is a genetic disease that causes increased levels of bradykinin, resulting in attacks of severe, rapid swelling of tissues that can last 3-5 days and may cause significant health problems and even death. Donidalorsen reduces production of pre-kallikrein (PKK) in hepatocytes, which in turn prevents the production of bradykinin, ultimately reducing swelling. Prior to the phase 2 study, patients had experienced an average of two attacks a month. During treatment with donidalorsen, after the second dose, there was a 97% reduction in attacks and all but one patient were completely attack-free. 17 of the 20 patients joined the open-label extension in which patients were dosed every four weeks over 13 weeks, then the dose could be adjusted to either a higher dose every 4 weeks or the same dose every 8 weeks, based on the physician’s recommendation. Donidalorsen was well-tolerated and maintained a good safety profile. 14 patients completed the entire 2 year OLE and experienced a mean 96% reduction in HAE attacks from baseline. Patients enjoyed 99.7% of days attack-free while being treated, and the 8-week dosing schedule also proved to be effective. Ionis has begun OASIS-HAE, a phase 3 study that will last 24 weeks and is already fully enrolled. Patients will have the option to join the OLE for a total of up to 3 years.
Concluding Remarks
We would like to express our profound appreciation to OTS President David Corey, Ph.D. and the Board of Directors for your leadership and dedication to the OTS Community.
We are incredibly grateful to this year’s meeting Co-Chairs, Alex Garanto, PhD and Punit Seth, PhD and the organizing committee, Pinar Akcakaya, PhD, Jillian Belgrad, MD, Rob Collin, PhD, David Corey, PhD, Keith Gagnon, PhD, Bruno Godinho, PhD, Aurelie Goyenvalle, PhD, Alan Horsager, PhD, Vasant Jadhav, PhD, Krystal Johnson, PhD, Art Krieg, MD, Holly Kordasiewicz, PhD, Erin McConnell, PhD, Rebecca Miles, PhD, Anna Perdrix Rosell, PhD, and Takanori Yokota, PhD. We appreciate all your efforts that produced a phenomenal meeting!
Thank you to everyone who spoke or submitted a poster. We truly appreciate your contributions to science and the time and effort invested into sharing your work with us. Finally, thank you to all the sponsors and exhibitors, and all who joined us in Spain or attended virtually. Your involvement and enthusiasm contribute to exceptional OTS Meetings!
We hope to see you at next year’s Annual Meeting in Montreal, Canada!








