
A divalent siRNA chemical scaffold for potent and sustained modulation of gene expression throughout the central nervous system
Alterman JF, Godinho BMDC, Hassler MR, Ferguson CM, Echeverria D, Sapp E, Haraszti RA, Coles AH, Conroy F, Miller R, Roux L, Yan P, Knox EG, Turanov AA, King RM, Gernoux G, Mueller C, Gray-Edwards HL, Moser RP, Bishop NC, Jaber SM, Gounis MJ, Sena-Esteves M, Pai AA, DiFiglia M, Aronin N, Khvorova A.
Joint First Authors

Julia F. Alterman

Bruno M.D.C. Godinho

Matthew R. Hassler

Chantal M. Ferguson
Currently, delivery to tissues other than the liver is the biggest challenge for oligonucleotide therapeutics. Even in encapsulated tissues like the central nervous system (CNS) where local administration is possible, negatively charged siRNAs are not taken up well by cells unless conjugated to hydrophobic ligands. However, this limits distribution within the brain.
For a more detailed discussion of the issue, watch Professor Anastasia Khvorova present this work at the recent HDSA Annual Convention:
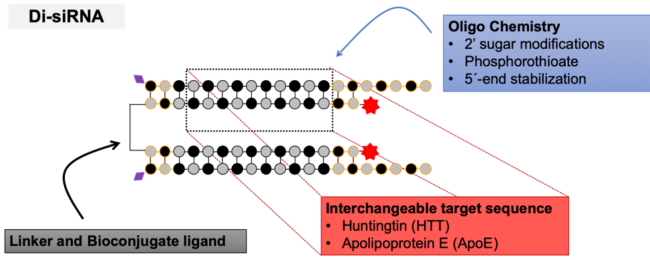
To solve the issue, Alterman, Godinho, Hassler, Ferguson and colleagues from the University of Massachusetts Medical School developed a new siRNA architecture termed divalent siRNA (di-siRNA). These di-siRNAs support widespread distribution and long-term silencing in the central nervous system of mice and nonhuman primates. They contain two 20-base guide strands hybridized with 15-base passenger strands with alternating methyl and fluoro 2′-modifications, two phosphorothioates at both ends plus five on the guide strand overhang as well as 5′ Vinyl phosphonate on the guide and Cy3 fluorescent labels on the passenger strands. The two siRNA passenger strands are covalently linked via a tetraethylene glycol linker on their 3′ ends.
Intracerebroventricularly (ICV) injection of di-siRNAs targeting the mouse huntingtin lead to broad distribution in the brain which was correlated with huntingtin (HTT) mRNA and protein downregulation. Similar results were achieved when targeting cyclophilin B and apolipoprotein E. A single high dose (475 μg) resulted in more than 90% reduction in HTT protein in the hippocampus and ~50% in the thalamus and striatum six months later. Di-siRNAs were also able to down-regulate both the wild-type mouse and mutant human HTT (97 CAG repeats) in the BACHD-ΔN17 mouse model. In non-human primates (NHPs), a single 25 mg ICV injection in the lateral ventricle lead to uniform distribution throughout the brain at 48 hours, with delivery to both neurons and glial cells. One month after injection, protein levels were reduced by more than 90% in the cortex, more than 80% in the hippocampus and around 50–85% in the caudate. With the exception of cervical white matter, di-siRNAs distributed well into and were functional in all cell types within the spinal cord, including neurons. Neither mice nor NHPs showed indications of inflammation, significant upregulation of IBA-1 and GFAP or changes in blood chemistry and cell counts. RNA-seq analysis showed minimal transcriptional changes or off-targeting activity.
Why you should read it
The paper demonstrates long-term efficacy and wide distribution of siRNA-based gene silencing in the CNS.






