The 14th Annual Meeting of the Oligonucleotide Therapeutics Society
September 30- October 3, 2018
Marriott Seattle Waterfront
Seattle, WA, USA

- RNAi
- Antisense
- mRNA
- CRISPR
- miRNA
- Aptamers
- Delivery
- Chemistry
- Safety
- Steve F. Dowdy, PhD, UCSD School of Medicine
Organizing Committee:
- Annemieke Aartsma-Rus, PhD, Leiden University Medical Center
- David Corey, PhD, UT Southwestern
- Masad Damha, PhD, FCIC, McGill University
- Richard Geary, PhD, Ionis Pharmaceuticals, Inc.
- Mark Kay, MD, PhD, Stanford University
- Anastasia Khvorova, PhD, RNA Therapeutics Institute, UMass Medical School
- Matthew Levy, PhD, Vitrisa Therapeutics
- Marc Lemaitre, PhD, ML_Consult
- Art Levin, PhD, Avidity Biosciences
- Mano Manoharan, PhD, Alnylam Pharmaceuticals
- Melissa J. Moore, PhD, Moderna Therapeutics
- Laura Sepp-Lorenzino, PhD, Vertex Pharmaceuticals, Inc.
- Takanori Yokota, PhD, Tokyo Medical and Dental University
Chemistry of Oligonucleotide Therapeutics 101
Mano Manoharan, PhD, Alnylam Pharmaceuticals
Oligonucleotides as a New Therapeutic Class: Progress Report & Prescription
Paul Burke, PhD, Burke Bioventures LLC
Unraveling the Mechanisms of ASO Mediated Toxicity
Sebastien Burel, PhD, Ionis Pharmaceuticals
Screening for Immunostimulation
Arthur M. Krieg, MD, Checkmate Pharmaceuticals
Regulatory Applications of a Nonclinical Database for Oligonucleotide Therapeutics at U.S. FDA
Emily Place, PhD, MPH, U.S. Food and Drug Administration
How a Drug is Assessed Once It’s in Clinical Trials: A perspective from a Clinical Pharm Reviewer
Bart Rogers PharmD, PhD, Office of Clinical Pharmacology, U.S. FDA
Q&A Panel (part of educational workshop)
Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis
Alejandra Gonzalez-Duarte, MD, National Institute of Medical Sciences and Nutrition–Salvador Zubiran, Mexico
2018 Annual Meeting – Welcome
Welcome & Opening Remarks
OTS President: Arthur M. Krieg, MD, Checkmate Pharmaceuticals Event Chairperson: Steven F. Dowdy, PhD, University of California, San Diego
2018 Annual Meeting Keynote
Keynote Presentation: Genome Editing Technologies and Beyond
Feng Zhang, PhD, Broad Institute of MIT and Harvard
2018 Annual Meeting Award Winner Presentations
Dr. Alan M. Gewirtz Memorial Scholarship Award Aiming for Ideality in the Age of Biomolecules
Justine N. deGruyter, The Scripps Research Institute Kyle Knouse, The Scripps Research Institute
Young Investigator Award Development of Nanomaterials for mRNA Therapeutics and Genome Editing
Yizhou Dong, PhD, The Ohio State University
2018 Annual Meeting Lifetime Achievement
Lifetime Achievement Award Chemical Synthesis DNA/RNA and Biological Activity of Selected Analogues
Marvin H. Caruthers, PhD, University of Colorado
Sleepless in Seattle (at least for this jet-lagged European)
The 14th annual meeting of the Oligonucleotide Therapeutics Society took place at the Marriott Seattle Waterfront hotel in Seattle, USA, from the 30th of September to the 3rd of October. With nearly 600 attendees and 150 posters, it was the biggest OTS meeting yet. Perhaps it was the Society’s increased social media engagement and educational efforts (E.g.Twitter @OTSociety, Local delivery program and webinars) that spread the word? Or maybe it was the widely publicised approval of ONPATTRO (the first siRNA drug) at the end of August or the “Spinraza” effect (the first oligonucleotide therapeutic to achieve blockbuster drug status)? Or perhaps the CRISPR and mRNA therapy sessions attracted people that would not normally attend? The field is clearly experiencing an upswing – truly the dawning of the age of oligonucleotide therapeutics!

Before going on, let me just say that the quality of talks was very high and obviously I can’t cover all of them, so these are some personal highlights.
Pre-conference educational session
As usual for OTS meetings, there was a pre-conference educational session which was recorded and these videos are available on the OTS website here. Mano Manoharan (Alnylam) started off the session with a general introduction to the field using the examples of the FDA approved oligonucleotide therapeutics fomivirsen (Vitravene, antisense), pegaptanib (Macugen, aptamer), mipomersen (Kynamro, gapmer antisense), eteplirsen and nusinersen (Exondys 51/Spinraza, splice modulating) as well aspatisiran (ONPATTRO, siRNA) to illustrate the advances in oligonucleotide chemistry and delivery.
Delivering delivery
Paul Burke (Burke Bioventures) expanded on this but taking us into the future. He pointed out that oligonucleotide drugs cover a much vaster therapeutic space than antibodies as they can target DNA and mRNA in addition to proteins. However, he cautioned that of the >20 RNAi drug candidates listed in a 2012 review, only one had been approved so far. He observed that there is a significant lag between research into delivery and clinical application which largely limits the current therapeutic opportunities to the liver and central nervous system. This theme of the need for improved delivery systems recurred through many of the main conference sessions.
Screening for inflammatory responses
Of interest in Sebastian Burel’s (Ionis) talk was the explanation of how Ionisscreen for TLR9 mediated inflammatory responses to antisense oligonucleotides. He referenced his 2017 paper on the subject, but also mentioned a B-cell derived cell line which shows particularly high TLR9 responsiveness. This cell line is especially sensitive to nucleic acid stimulation of the cellular immune response and can be used as a more convenient replacement for human PBMCs. He promised that he would be publishing about this new assay soon.
Art Krieg (Checkmate Pharmaceuticals) expanded on the issue of the immune system in a more practical manner with emphasis on species differences between mouse and human, how to test for immune activation, the proper timepoints for measuring cytokines (3-12 h, unlikely to be > 24 hours) and the importance of assay conditions (cell number, round-bottom plates for hPBMCs). See also his webinar from earlier this year.
 Regulators perspective on oligonucleotide therapies
Regulators perspective on oligonucleotide therapies
Two FDA representatives presented their personal views. Emily Place (FDA) gave an overview of the accumulated data on oligonucleotide therapies FDA holds in their internal database. So far, they have not identified any genotoxicity safety signals with oligonucleotide therapeutics. Hobart Rogers (FDA)then presented on how a drug is assessed by the FDA with lots of practical advice for the researcher (note the innovative use of pasta shapes to illustrate the essential differences between single-stranded antisense and double-stranded siRNA molecules – see picture).
 Question and Answer session with all the speakers followed.
Question and Answer session with all the speakers followed.
Next Generation Early Career Scientist Session
During the lunch break, Maire Osborn (Khvorova lab, now Dicerna) and Shruti Sasaki (Ionis), the OTS traineeBoard representatives, had organised the NextGen Early Career Scientist Session. This session consisted of a keynote address by Jason Shepherd (University of Utah)and 7 short talks by early career scientists who were competing for the top spot as voted by their peers. The session was fully booked, with standing room only. Out of the impressive selection, “Harnessing Endogenous ADARs for Transcript Repair” by Tobias Merkle (University of Tübingen) was voted the winner of the best talk.
Keynote Presentation
The main conference began with the keynote address given by Feng Zhang (Broad Institute of MIT and Harvard) on “Genome Editing Technologies and Beyond”(this lecture as well as the award lectures on Wednesday were also recorded and are available on the website). After a brief general introduction to gene editing, he quickly concentrated on the novel CRISPR effector Cas13 which can target viral ssRNA. In the presence of target RNAs, Cas13 will be activated to also cleave additional RNAs. In this way, a bacterial cell overwhelmed by a viral infection can commit altruistic suicide to prevent spread of the virus. Cas13 can also be used to detect nucleic acids, such as viral sequences in simple tests similar to pregnancy tests, while inactivated dCas13 can target RNA-editing enzymes such as ADAR to specific sequences for targeted RNA editing.
Session I: Nucleic Acid Chemistry
The first session started off with an excellent talk by Punit Seth (Ionis Pharmaceuticals) who demonstrated how the gap region of antisense oligonucleotides is a key region for interaction with cellular proteins. He used the crystal structure of RNaseH1 and RNaseH1 cleavage patterns of ASOs bearing various chemical modifications at specific positions in the gap region to illustrate this concept. In particular, he showed how FANA, FHNAand neutral backbone modifications increase allele-specificity and ASO selectivity depending on the position of these modifications within the gap.
 Daniel Anderson (MIT) and Daniel Siegwart (University of Texas) presented on their work with lipid nanoparticles for siRNA, mRNA and CRISPR/Cas9 delivery. Anderson showed examples of successful delivery for each, with the most impressive the functional delivery of luciferase mRNA to the lung endothelium using inhaled nanoparticles containing PBAE, DOPE, PEG-lipid and cholesterol. Siegwart meanwhile concentrated more on the differences in the compositions of his zwitterionic amino lipids, with data on delivery of fumarylacetoacetate hydrolase mRNA to knockout mice. The subject of delivery, be it ASO, siRNA, mRNA or CRISPR/Cas systems certainly was a hot topic and wound itself through most of the sessions.
Daniel Anderson (MIT) and Daniel Siegwart (University of Texas) presented on their work with lipid nanoparticles for siRNA, mRNA and CRISPR/Cas9 delivery. Anderson showed examples of successful delivery for each, with the most impressive the functional delivery of luciferase mRNA to the lung endothelium using inhaled nanoparticles containing PBAE, DOPE, PEG-lipid and cholesterol. Siegwart meanwhile concentrated more on the differences in the compositions of his zwitterionic amino lipids, with data on delivery of fumarylacetoacetate hydrolase mRNA to knockout mice. The subject of delivery, be it ASO, siRNA, mRNA or CRISPR/Cas systems certainly was a hot topic and wound itself through most of the sessions.
A very well attended poster session combined with a welcome reception followed the session (our board members Paloma Giangrande and Jonathan Watts were certainly enjoying themselves). And if that wasn’t enough to tire you out, there was an Early Career Scientist Socialat a local bar. By that time however, I was so tired that I was barely able to string coherent English sentences together!
Session II: Delivery
The next day started with a stellar talk on “CNS active RNAi”by Anastasia Khvorova (University of Massachusetts Medical School).Prof. Khvorova demonstrated how full metabolic stabilization of siRNA is essential for effective CNS delivery. She showed that siRNA hydrophobicity and PS content define brain retention and distribution such that conjugation with lipophilic ligands can increase retention from minimal to good. SiRNAs without PS modifications equal PBS for lack of retention, yet PS content drives both retention AND toxicity. To address this issue, Khvorova developed Di-siRNAs – two siRNAs connected by a linker on the 3′ ends of their sense strands and with hydrophobic lipid moieties on the 5′ ends of the same strand. Huntingtin-targeted Di-siRNAs demonstrated persistence of mRNA downregulation in hippocampus, thalamus, striatum, posterior cortex and medial cortex of mice up to 6 months after a single 475 µg ICV injection. HTT protein was completely absent in all regions at 1 month and still significantly reduced in some regions at 6 months. In NHPs, HTT mRNA levels were reduced to 35% of untreated animals in cortex and hippocampus, 45% in caudate and 75% in putamen 1 month after a single CSF injection of 25 mg with congruent significant reductions in protein levels. Look out for the forthcoming paper on this or watch the excellent webinar Prof. Khvorova did for OTSwhich discusses some of her research!
In a change of schedule (and topic), Clive Meanwell (The Medicines Company) highlighted the truly impressive data from the inclisiran phase II trial in a talk starting with the phrase “Civilization kills” (by cardiovascular disease) and intermixed with musings on drug pricing, sustainability, and the need to expand production capacity for oligonucleotide therapeutics. Treating a projected 1.6 million people with inclisiran would require 6-10 tons of drug/year, while current maximum capacity is 7-10 kg/batch. Capacity increases are clearly needed and already in the works.
Food for thought there… perhaps new methods of enzymatic synthesis for modified oligonucleotides will offer a solution?
Session III: mRNA and CRISPR
These two topics are of course very hot at the moment, and delivery of these even more so. Prof. Paula Hammond (MIT) started off the session explaining how n3 and n5 polyamines can stabilize mRNA complexed to eIF4E and thus enhance mRNA expression. This approach can also be applied to siRNA-Ago2 or other RNA/protein complexes. The other talk I really liked in this session was from Eric T. Wang (University of Florida), who told us about the use of dCasto impede transcription in microsatellite repeat expansion diseases such as Fuchs endothelial corneal dystrophy, myotonic dystrophy and C9orf72-ALS/FTD. He showed that (CAG)6 but not (AGC)6 or (GCA)6guide RNAs could block transcription in a length-,PAM-, and strand-dependent manner. An interesting concept!
An honourable mention to Zachary Kennedy (University of Massachusetts Medical School) who had the scientific integrity to show that the survival extension he achieved with CRISPR knockdown of SOD1 in an ALS mouse model was not the longest compared to previously published therapies.
An extended lunch break with optional “Meet the experts” round tables followed.
 Session IV: miRNAs and Aptamers
Session IV: miRNAs and Aptamers
Nebojsa Janjic (SomaLogic) delighted us with a quote from J.B.S. Haldane (see picture). He then elaborated on his work on using amino acid side chains to increase chemical diversity of aptamers, some of which I have covered here. Angelo Moreno (Duke University) gave a presentation on PEGylated aptamer inhibition by Anti-PEG antibodies, a talk highly relevant to anyone using PEG in human therapeutics. Apparently, 44% of healthy individuals have such antibodies. Not only are they the cause of the Pegnivacogen allergic reactions that led to termination of its clinical development, but they also decrease therapeutic efficacy of aptamers. This isn’t just an aptamer issue either! In the previous session Joe Senn (Moderna) showed how anti-PEG IgM antibodies inhibit delivery of PEG-containing mRNA LNPs to liver hepatocytes, and how they developed safer“faster PEG shedding” 3rd generation LNP vehicles.
 Time to find some viable alternatives to PEGylation!
Time to find some viable alternatives to PEGylation!
An interesting talk on the advantages of remlarsenin corneal fibrosis by Corrie Gallant-Behm (miRagen) finished off the session (accompanying press release with data summary here). The second poster session afterwards (see picture) was as good if not better than the first one and again very well attended!
Session V: Awards Presentations & Talks
As these are available on the website, I will only say that the quality of the award winners reflected the increasing competition in the field. The Lifetime achievement award winner Marvin Caruthers (University of Colorado) is in a class of his own, and I will cover his contributions to the field in a separate piece.
Session VI: Target Identification, Drug Discovery and Trial Design
A catch-all title for a very varied but nevertheless interesting session. Martin Maier (Alnylam– see presentation here) and Mariam Aghajan (Ionis) updated us on how improvements in chemistry have reduced the off-target potential of siRNAs and increased their therapeutic index 5-fold (Maier) and allowed for development of an ASO that down-regulates both liver and kidney APOL1 to treatAPOL1-associated nephropathy (Aghajan). Isabel Aznares (Stoke Therapeutics) then familiarized us withStoke’s unique way of increasing gene expression by reducing naturally occurring non-productive alternative splicing events (not disease-causing splicing events). In a heterozygous mouse model of Dravet Syndrome this interference could increase NaV1.1 protein level after a single bolus injection and this persisted for more than 90 days.
This was followed by Amit Deshwar (Deep Genomics), who told us how their updated computer program can rapidly identify therapeutic targets for splice switching oligonucleotides. Last, but not least, Laurence Gainey (HGF) gave us 7 tips for getting a robust patent using the example of Terradactyl Technologies’ patent application for the wheel. Also see the webinar Dr. Gainey did for OTS on this subject. He has a gift for distilling down what a researcher should know and do (or avoid) about patenting.
 We got the afternoon off to socialise or explore Seattle and then there was the party! Geri and Alexis (Event Innovations), our event organisers, managed to top last year’s fantastic party at a wine-producing Chateau outside Bordeaux by booking the Seattle Museum of Pop Culture. You could explore the exhibits, have some food and drink, chat or dance in front of a massive screen (showing the Space needle in the picture – MoPOP is right next to it!).
We got the afternoon off to socialise or explore Seattle and then there was the party! Geri and Alexis (Event Innovations), our event organisers, managed to top last year’s fantastic party at a wine-producing Chateau outside Bordeaux by booking the Seattle Museum of Pop Culture. You could explore the exhibits, have some food and drink, chat or dance in front of a massive screen (showing the Space needle in the picture – MoPOP is right next to it!).
I didn’t stay very long as I was still fighting the jet-lag, but from all accounts it was a fantastic party (also see the #OTSSeattle twitter feed)
Session VII: Preclinical development
Before starting, the incoming president of OTS, Annemieke Aartsma-Rus announced the Poster Award Winners – you can find a list here.
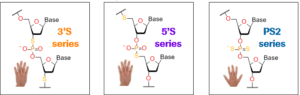 “Expanding the Drug Property Space of Locked Nucleic Acids through (thio)phosphate modifications” by JörgDuschmalé (Roche Innovation Centre Basel) was another great talk. Leading on from Marvin Caruther’sLifetime Achievement Award lecture, he told us about the use of thiophosphate modifications in stereo-defined LNA gapmer oligonucleotides to reduce diastereoisomericdiversity. He showed that 3′ and 5′ thiophosphate backbone modifications (see picture) perform poorly due to limited stability against nucleasedegradation, but that up to 4 non-chiral phosphorodithioatesin combination with stereo-defined phosphorothioate linkages can improve in vivo potency. A great talk with the chemistry so well explained that even chemically challenged me could follow it! Stereochemistry is of course a hot topic in the field, so there was another talk on this topic by Chandra Vargeese (Wave) in the next session (I covered some of Wave’s work here).
“Expanding the Drug Property Space of Locked Nucleic Acids through (thio)phosphate modifications” by JörgDuschmalé (Roche Innovation Centre Basel) was another great talk. Leading on from Marvin Caruther’sLifetime Achievement Award lecture, he told us about the use of thiophosphate modifications in stereo-defined LNA gapmer oligonucleotides to reduce diastereoisomericdiversity. He showed that 3′ and 5′ thiophosphate backbone modifications (see picture) perform poorly due to limited stability against nucleasedegradation, but that up to 4 non-chiral phosphorodithioatesin combination with stereo-defined phosphorothioate linkages can improve in vivo potency. A great talk with the chemistry so well explained that even chemically challenged me could follow it! Stereochemistry is of course a hot topic in the field, so there was another talk on this topic by Chandra Vargeese (Wave) in the next session (I covered some of Wave’s work here).
On a different note, David Blakey (MiNa) updated us on the progress of the small activating RNA (saRNA) MTL-CEBPA clinical trial in patients with advanced liver cancer. They found an interesting synergy with the tyrosine kinase inhibitor sorafenib (see press release) with two patients experiencing confirmed complete tumour responses with sorafenib subsequent to treatment with MTL-CEBPA. I have previously covered saRNAhere and here. In my opinion saRNA is an interesting area of oligonucleotide therapeutics with numerous potential applications that seems to be very much overlooked!
Maja Janas (Alnylam– see presentation here) then told us about the safety of 2′-F-monomer metabolites from 2′-F-modified nucleotides in GalNAc-siRNA conjugates. She showed that 2′-F-monomers are poor polymerase substrates, do not seem to inhibit polymerase, do not cause chain termination, and do not impact mitochondrial function with chronic dosing (97 weekly doses of 100 mg/kg in rat). Robert MacLeod (Ionis) presented pre-clinical data (mouse & NHP) on IONIS-IRF4RX for the treatment of B cell malignancies, demonstrating the use of Ionis Generation 2.5 chemistry in targeting B cells. Yutaka Kondo’s (Nagoya University) talk on using antisense oligonucleotides to target the noncoding RNA TUG1 as a treatment for glioma was interesting as they took advantage of the enhanced permeability of the blood brain barrier caused by the disease to effectively deliver the ASO in unit polyion complexes or peptide-conjugated polymeric micelles (see the paper here).
Session VIII: Preclinical II
Xiang-Dong Fu (University of California) presented his work on reprogramming astrocytes into dopaminergic neurons using either AAV-shPTB or PTB-ASO. These reprogrammed neurons were able to reverse chemically induced Parkinson’s Disease in mice. What an interesting strategy!
Adrien Weingärtner (Silence Therapeutics– see presentation here) disclosed their optimization work on serinol-linked GalNAc clusters for siRNA delivery. He showed that 5′ antisense conjugates are stable but inactive, and 2-4 serial GalNAc units showed little difference compared to triantennaryGalNAcs while single GalNAcs at the 3′ and 5′ end of the siRNA sense strand increased stability and in vivo activity. ThisGalNAcdesign should be easily achievable for academic labs!
Session IX: Clinical Studies
This session started off with an update on the Givosiran open label extension study (Amy Simon, Alnylam), followed by a summary of the Ionis/Akcea cardiometabolic disorder clinical trials (Richard Geary, Ionis) and an overview of the ongoingLumasiranphase I/II trial(Patrick Haslett, Alnylam). Anna Collén (AstraZeneca) then told us about the use of VEGF-A mRNA to treat cardiac disease. Taking advantage of the transient expression of mRNA therapies to limit VEGF-A exposure, they showed that AZD8601 increased blood vessel formation, blood flow and cardiac function after myocardial infarction in mice and pigs. A phase 2a study in patients undergoing coronary artery by-pass grafting is ongoing.
I really like it when someone takes something everyone views as a disadvantage and turns it into a positive!
John Kirkwood (UPMC Hillman Cancer Center) then took us through the data from the CMP-001 + pembrolizumab in PD-1 resistant melanoma phase I trial (see press release). Oligonucleotide TLR9 agonists in combination with anti‐PD‐1/PD‐L1 therapeutics is a hot topic with a number of other companies running clinical trials. CMP-001 is unique in that it is a CpG-A type encapsulated in a virus‐likeparticle (VLP)and differs from other CpG classes in clinical development by having a native DNA backbone that induces the highest levels of type I IFN. Early days, but this is very promising!
Session X: Late-breaking Clinical Studies
This session included a very good talk, “Proof-of-concept for RNA editing Oligonucleotide QR-110 for Treatment of Inherited Retinal Dystrophy in Adults and Children with LCA10” by David Rodman (ProQR –see the press release). LCA10 Leber’s congenital amaurosis is due to a c.2991+1655A>G mutation in the CEP290 gene and typically leads to childhood blindness. In this trial, 60% of subjects showed a clinically meaningful response in visual acuity and mobility course endpoints at three months of treatment. The company is seeking to start a phase II/III trial that could serve as the sole registration trial in 2019. A very impressive end to the meeting!
An optional pub crawl followed – but the jet lag was still bothering me, so I opted for a quiet dinner to catch up with some friends.
 To finish off, I would like to make some general comments. It is a sign of the changing times that the research publication cycle has changed from presenting data at a meeting followed by publication in a paper to patenting first, followed by embargoed paper publication, and then meeting presentation. In one way this is positive, as it shows how the field has really moved into clinical translation, but we should strive to not lose our scientific roots and the spirit of collaboration that founded the Society.
To finish off, I would like to make some general comments. It is a sign of the changing times that the research publication cycle has changed from presenting data at a meeting followed by publication in a paper to patenting first, followed by embargoed paper publication, and then meeting presentation. In one way this is positive, as it shows how the field has really moved into clinical translation, but we should strive to not lose our scientific roots and the spirit of collaboration that founded the Society.
Also, I would like to thank our sponsors without whom this meeting would not be possible. Their contribution allowed OTS to again increase the number of travel grants for young scientists (22 this year – see here for the list of recipients).
I hope I have managed to convey that attending an OTS meeting is well worth it, not just for the talks but also for the opportunity to meet other scientists working in the field, start collaborations and perhaps find new challenges for your research. So, I hope to see you all next year at #OTSMunich! I wonder what Geri and Alexis have planned for the party?