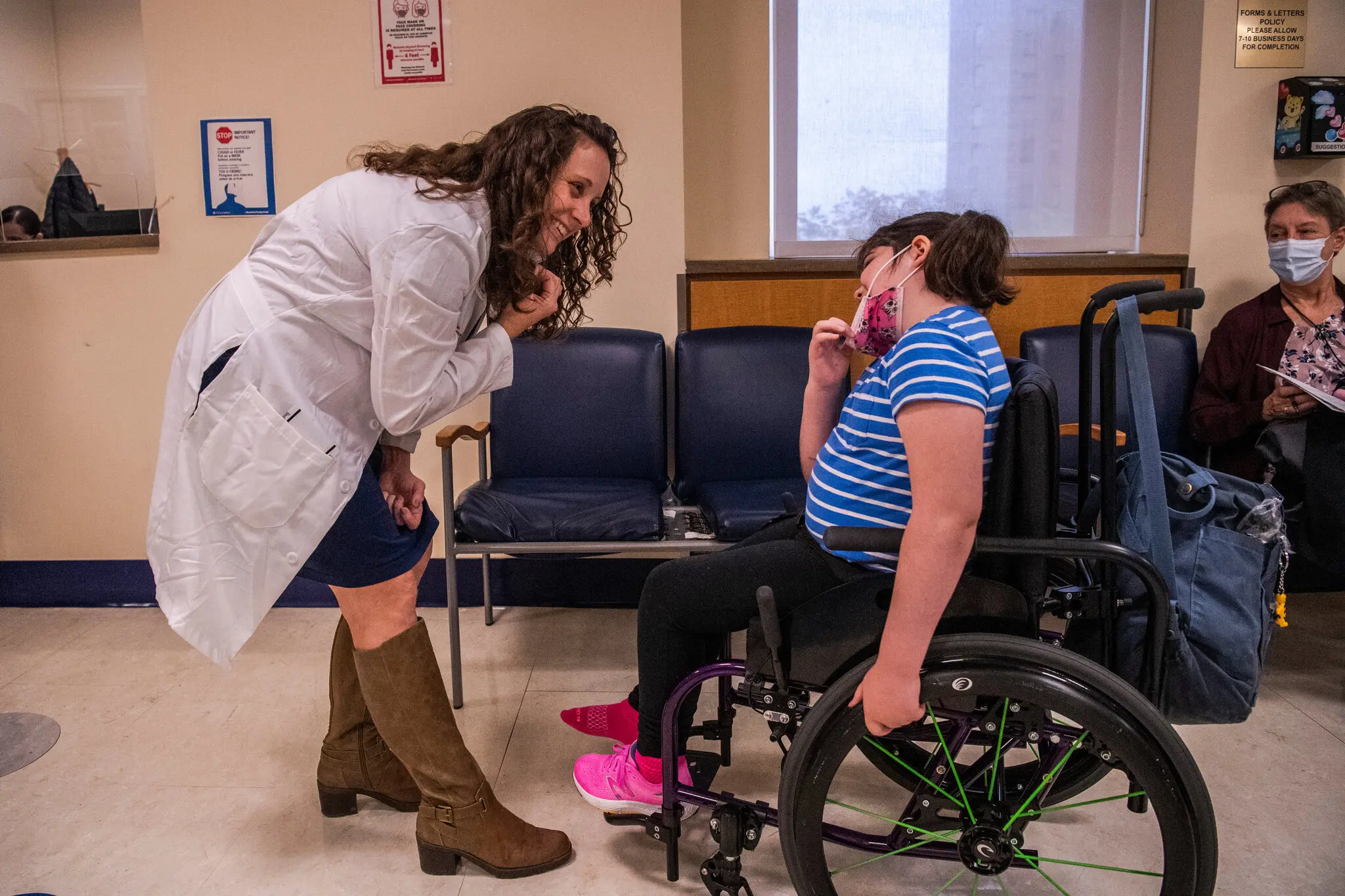
Looking for something?
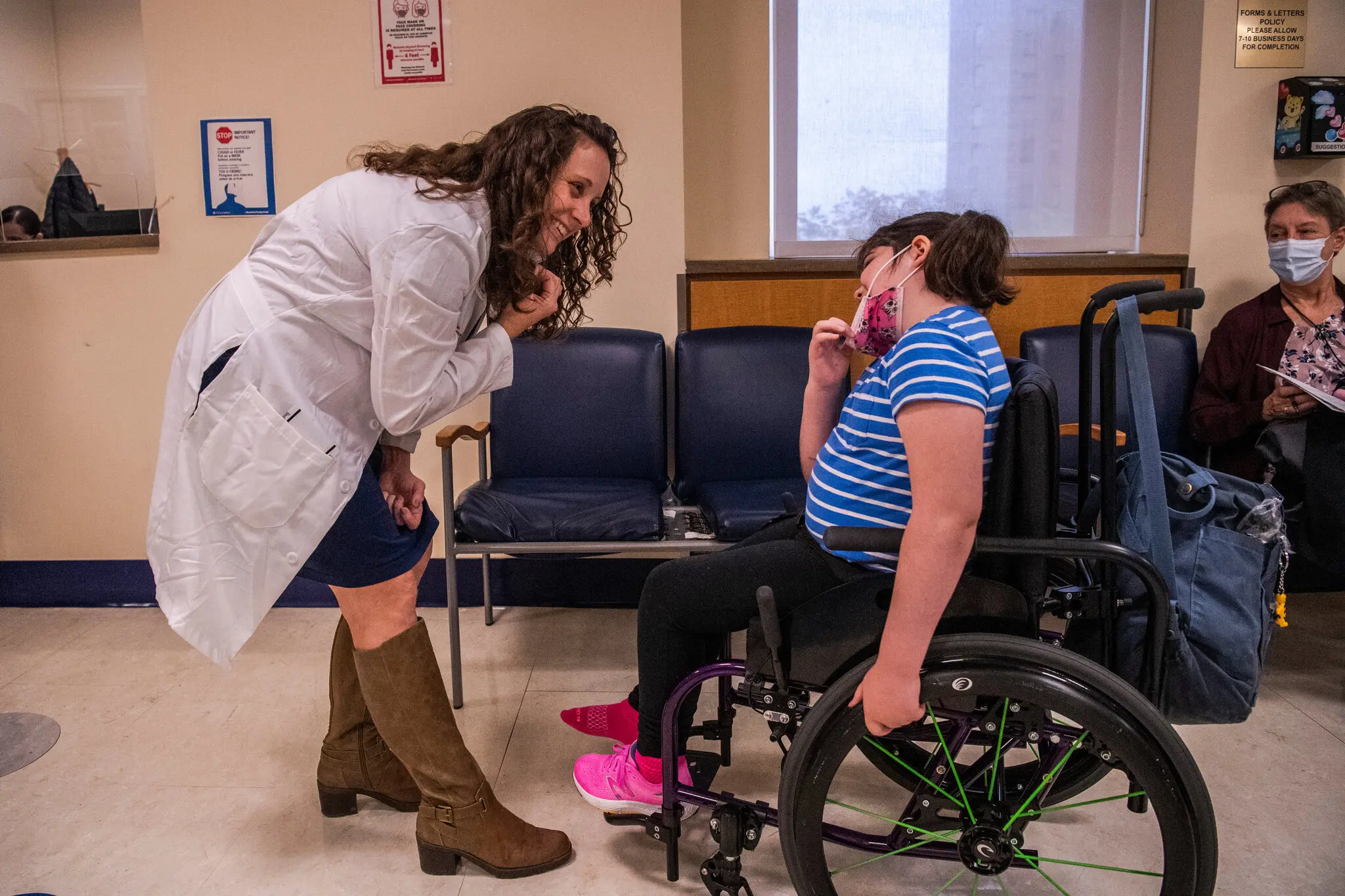
By Erika Check Hayden (New York Times)
Photographs by Brittainy Newman
Scientists have made rapid progress in customizing drugs for ultrarare diseases. The hard part now is making such treatments on a large scale.
Susannah Rosen, 8, spent much of her childhood in hospitals in New York City as doctors documented the gradual loss of her ability to stand, walk and see.
But on a visit this October, her parents thought for the first time that she might leave the hospital better off than before. That’s when surgeons infused a drug into her spine to fix the ultrarare genetic glitch that had vexed her nervous system since infancy.
“Every other time we go into the hospital, it’s because something terrible has happened,” said Susannah’s father, Luke Rosen. “This time, there was hope for something that will heal her.”
Susannah was the first person to receive a drug designed to treat KIF1A-associated neurological disorder, or KAND, a progressive disease caused by genetic mutations that affect just 400 people in the world. In doing so, the young girl and her parents have found themselves on the edge of personalized medicine.
Since the technology for such bespoke genetic drugs debuted in 2018, about two dozen patients have received the infusions — costing as much as $2 million per patient — to treat a range of neurological syndromes. But hundreds of millions of others, mostly children, live with rare genetic diseases and have no treatment options.
Susannah’s drug, almost two years in the making, was paid for by a nonprofit organization, n-Lorem, that aims to do the same for at least 1,000 patients over the next decade. By raising funds and negotiating discounts and in-kind donations from biotechnology companies to make its drugs, n-Lorem’s founder believes, the organization can fulfill its mission to “leave no child behind.”


