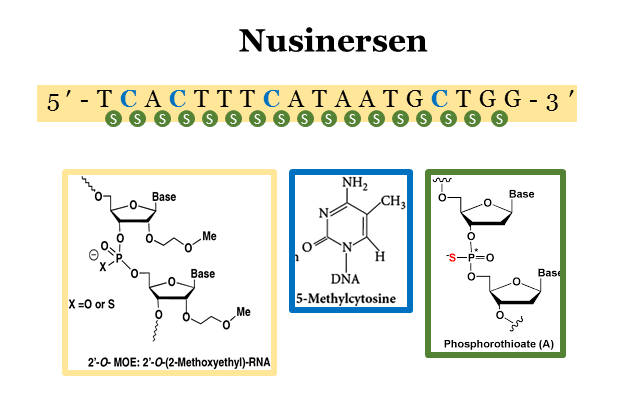

Nusinersen Initiated in Infants During the Presymptomatic Stage of Spinal Muscular Atrophy
De Vivo DC, Bertini E, Swoboda KJ, Hwu WL, Crawford TO, Finkel RS, Kirschner J, Kuntz NL, Parsons JA, Ryan MM, Butterfield RJ, Topaloglu H, Ben-Omran T, Sansone VA, Jong YJ, Shu F, Staropoli JF, Kerr D, Sandrock AW, Stebbins C, Petrillo M, Braley G, Johnson K, Foster R, Gheuens S, Bhan I, Reyna SP, Fradette S, Farwell W; NURTURE Study Group.
Joint First Authors

Sandra P Reyna

Nancy Kuntz

Wuh-Liang Hwu

Russell Butterfield
Senior Authors

Monique Ryan

Tawfeg Ben Omran

Douglas Kerr

Darryl C. De Vivo
Nusinersen (Spinraza) for the treatment of Spinal Muscular Atrophy (SMA) has been a breakthrough for antisense oligonucleotides. Click here for some background on SMA and a summary of the clinical development program or here for an explanation of how Spinraza works. Here, De Vivo and colleagues report interim efficacy and safety data from the ongoing Phase 2 NURTURE (NCT02386553) trial in presymptomatic children with SMA (data cut-off March 2019).
Watch Prof. Richard S. Finkel discuss this data:
The ongoing trial evaluates treatment with nusinersen in fifteen children with two SMN2 copies and ten with three SMN2 copies. At the time of the interim analysis, all participants were alive, and none required tracheostomy or permanent ventilation, though 4/15 children with two SMN2 copies had needed respiratory support for ≥6 h/day for ≥7 consecutive days during the study. Only two of these children still require respiratory intervention for 2h and 10 hours per day, respectively.
However, all 25 children are able to sit without support, 23/25 are walking with assistance, and 22/25 without assistance. The non-walkers are from the group with only two SMN2 copies. Motor function also increased significantly, approaching, or achieving the maximum of 26 points on the HINE-2 and 64 points on the CHOP INTEND scale. Seven of 15 infants with two SMN2 copies presented with protocol-defined symptoms of SMA at 24 months of age but their motor function continued to improve, while none of the infants with three SMN2 copies had symptoms. Children with three SMN2 copies showed better functional outcomes than those with two copies.
Why you should read it
Nusinersen continues to deliver outstanding patient benefit, with treatment of presymptomatic children leading to significantly better clinical outcomes than later treatment. This paper makes a very strong case for inclusion of SMA testing in new-born screening.






