Looking for something?
Lipid Nanoparticles: Nanomedicine’s Triumph
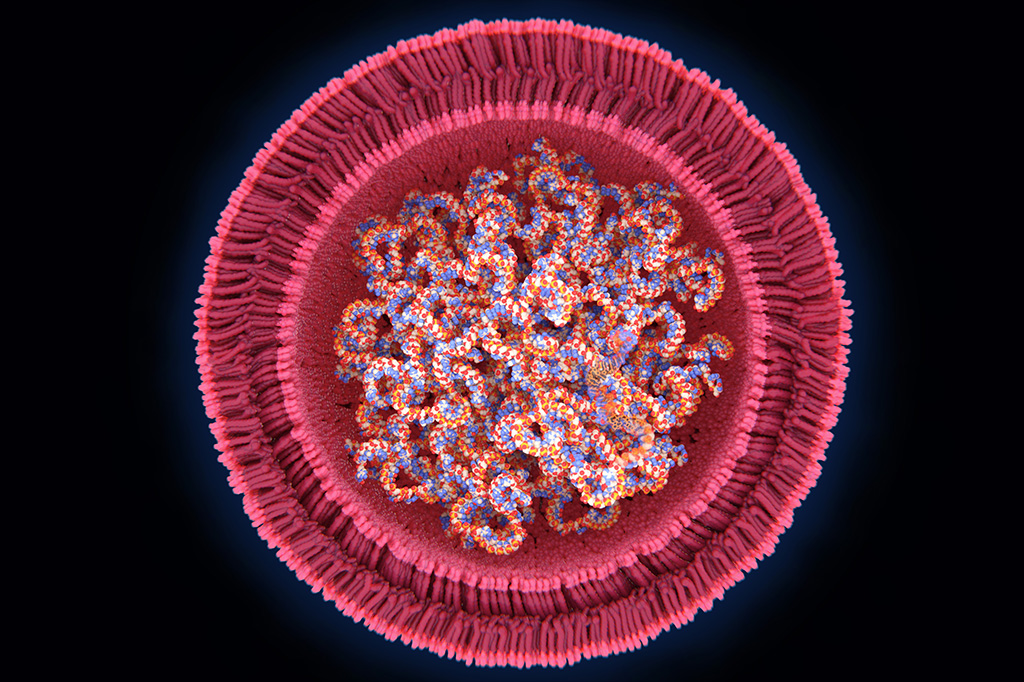
Oligonucleotide therapeutics have been making headlines this past year, between the groundbreaking new mRNA vaccines and the recent news of phenomenal interim results for Intellia’s investigational genome editing treatment for ATTR amyloidosis. Neither of these would have been possible without a lesser-known component – lipid nanoparticles. This delivery system has been decades in the making and, fortunately for humanity, was developed in time to play a role in saving millions of lives.
Lipid nanoparticles (LNPs) are an advanced delivery system that arose from the use of liposomes, which are a simpler delivery system. Liposomes are hollow spheres of lipids that drugs diffuse into. The liposomes can safely carry the drug into target cells and release it. The first FDA approval of a drug that utilized liposomes was given to Doxil, a chemotherapy drug (doxorubicin) enclosed in a liposome which reduced cardiotoxicity and improved anticancer ability compared to administration of free doxorubicin (1). While liposomes are useful in delivering drugs that range from cancer treatment to pain relief (1), improvements to this system needed to be engineered to begin offering the possibility of delivering nucleic acids.
Lipid nanoparticles (LNP) are one of the most advanced non-viral delivery systems (2, 3) and multiple LNP drugs have received approval by regulatory agencies (2). Scientists have been attempting to use various lipid-based nanoparticle formulations to create vaccines for viral infections and tumors for nearly three decades (4).
For many therapies, LNPs are an excellent choice to deliver the cargo as they address many of the necessary aspects required of a nucleic acid delivery vehicle. A carrier is necessary to protect the nucleic acids from being degraded and a sophisticated delivery system is necessary to enable the nucleic acids to survive long enough to reach the target cells. Once it reaches the cell, the particle must be transported through the cell membrane (through endocytosis in this case). Inside the cell, the cargo needs to be released from the carrier into the cytoplasm where it can finally do the job it was created for.
This may seem like a straightforward sequence, but the process is incredibly complex. Groups that persisted in the painstaking work of developing usable formulations had to overcome a plethora of barriers in addition to those already mentioned. Any particle formulations need to be stable in storage, and in the human body (1). An efficient delivery system must also carry sufficient amounts of the nucleic acid (1), degrade quickly so it does not accumulate in tissues, and not be toxic (3, 5) while also evading immune clearance (6).
Even the optimal size for the particle and the best way to create LNPs had to be determined. Often, lipids dissolved in ethanol are mixed with nucleic acids dissolved in an acidic buffer. When the two solutions are merged in a rapid mixing production technique, they spontaneously form LNPs that are 60 to 100 nanometers in size (7). To put that into context – 1 million nanometers fit into a single millimeter.
Optimal components to form the LNP also had to be determined. The lipids used are particular types that are present in the cell membranes in the human body. LNP delivery systems for mRNA and siRNA contain four parts. These are typically ionizable lipids, PEGylated lipids, phospholipids and cholesterol lipids (which help form the structure of the nanoparticle). Thousands of these lipids make up one particle (1, 6, 7, 8).
PEGylated lipids are composed of lipid heads attached to polyethylene glycol (PEG). They are a vital component of LNPs because they protect the particle from being detected by the immune system. They also help control particle size while it is forming and prevent particles from aggregating (clumping) together while in storage.
All this may sound simple, but it took years of engineering to find methods that produced consistent results, and formulations that were not toxic, degrade quickly enough that they would not accumulate with repeated doses, and actually enter target cells.
A final concern is that nucleic acids are negatively charged, so need to be balanced by positively charged lipids. However, permanently positively charged lipids are too toxic to cells to provide a solution.
A very interesting solution to this last concern was found – lipids that are positively charged under certain conditions. The right formulation was engineered that allows the LNP to be positively charged in acidic conditions in order to bind to and encapsulate RNA, while neutral at physiological pH to reduce toxicity while circulating in blood (1, 5, 6, 9). Then, once bound to the cell, the nanoparticle is encapsulated in an endosome, which has an acidic internal environment. This causes the ionizable lipids to become positively charged and changes the shape of the LNP. After the shape change, the LNP breaks out of the endosome and releases its cargo into the cytoplasm (1, 10).
In attempts to make new LNPs that could meet all the demands required to create an efficient, nontoxic delivery system for siRNA, Alnylam formed partnerships to make new LNPs. In partnership with Protiva Biotherapeutics and Inex Pharmaceuticals, more than 300 ionizable lipids were engineered. They optimized the tails, ionizable head group, and linker region, in addition to many other factors. Many formulations that worked and delivered their cargo to cells in culture failed in animal studies (10). However, once ionizable cationic lipids were optimized they delivered remarkable improvements in gene silencing potency (2).
MC3 is the ionizable lipid that Alnylam ultimately developed and used in Patisiran (Onpattro) to deliver siRNA to hepatocytes. Not only was Patisiran the first siRNA drug to receive FDA approval, but it was also the first drug to be approved that used LNPs as a delivery vehicle (11). Despite this success, the field moved toward chemical conjugations and other methods of safely and effectively delivering siRNA (10).
Both Moderna and BioNTech decided to use the knowledge of LNPs that was discovered in creating delivering methods for siRNA in the development of mRNA therapies for the treatment of diseases and in vaccines. Existing formulations for siRNA did not work well to deliver mRNA, so they endeavored to find an optimal formulation for mRNA delivery. One change discovered by Moderna was simply in altering the ratio of the four lipids in the nanoparticles. The modifications they made changed distribution throughout the body and made the LNP more biodegradable (10).
Moderna and BioNTech’s investments paid off since they had usable LNPs ready when COVID-19 exploded into a global pandemic. Without needing to formulate a new LNP, it was a relatively simple matter to replace the mRNA already being tested in vaccines with mRNA that codes for the SARS-CoV-2 spike protein to create a safe, effective mRNA vaccine.
Although both company’s LNPs have been used to deliver the vaccine to millions of people worldwide, LNP formulations can still be optimized for intramuscular injection of vaccines, which could result in a lower dose. A lower dose would allow for swifter manufacturing and broader distribution of vaccines in future pandemics (10).
In addition to Patisiran and the COVID-19 mRNA vaccines, many companies have entered candidates utilizing various LNP formulations into clinical trials, including Alnylam Pharmaceuticals, Arbutus Biopharma Corporation, Dicerna Pharmaceuticals, Arrowhead Pharmaceuticals, Moderna Therapeutics, BioNTech, Translate Bio, Arcturus Therapeutics, Nitto BioPharma, Chula Vaccine Research Center, Globe Biotech Limited, and Shulan (Hangzhou) Hospital; Center for Disease Control and Prevention. The therapies include vaccines and treatments for cancer, rare diseases, and infectious diseases (1).
Other companies have developed proprietary LNP platforms. One example is Arcturus Therapeutics, which developed an LNP platform called Lipid-enabled and Unlocked Nucleomonomer Agent modified RNA (LUNAR®) composed of proprietary ionizable amino lipids. The lipids degrade rapidly and are optimized for high RNA and DNA encapsulation efficiency. This platform can be modified to target specific cell types or tissues and can deliver small siRNAs and microRNAs and also larger mRNAs and gene editing technologies such as CRISPR and TALENs, or even mixtures of different RNAs.
Since LNPs can accommodate large payloads, they are also suitable for gene editing therapeutics (6). This ability was nicely displayed in Intellia Therapeutics’ early results of the genome editing treatment (NTLA-2001) for ATTR amyloidosis. NTLA-2001 consists of LNP encapsulating Cas9 mRNA and a single guide RNA which is delivered to hepatocytes where it results in the knockout of TTR protein, reversing disease progression.
LNPs have many advantages as delivery systems. They can carry large payloads and a wide variety of cargo, have structural design flexibility, multi-dosing capabilities, low immunogenicity, and are relatively easy to manufacture allowing for rapid, large-scale production (1, 2, 6).
While extraordinary milestones have been reached through the use of LNPs, there is still much room for improvement. Most notably, distribution to additional cells and tissues beyond immune cells, tumors, and the liver would allow for LNPs to deliver much broader applications.
Using different cholesterol molecules, phospholipids, and ionizable lipids could improve delivery and allow for delivery to different cells and tissues (10). Since the structure of the particle is instrumental in determining delivery, various optimizations provide opportunity to deliver different drugs and nucleic acids to a range of tissues. For example, nanoparticle size plays a role in delivery efficiency, as well as what tissues the particle will ultimately reach. Negatively charged LNPs target lymph nodes, while neutral LNPs are suitable for delivering chemotherapies, and positively charged LNPs have a strong cellular affinity and have higher immunogenicity (which is desirable in vaccines and undesirable for gene-silencing therapies). Optimal pKa is different for siRNA than for mRNA (1). PEGylation impacts clearance of particles from the bloodstream and can increase particle retention and uptake of particles by the targeted tissues and organs (1, 12). Groups around the world are resolutely working on these, and many other considerations, to improve and refine lipid nanoparticles.
Indeed, there have been quite a few notable recent advances in LNPs. The following examples only represent a fraction of the incredible discoveries that have been recently reported. We would love to hear about your work in a comment below.
Hirai et al. synthesized a novel charge-reversible lipid derivative. This LNP is a highly stable and potent siRNA delivery vector and enhances uptake into cancer cells (13).
Zukancic et al. discovered innovative PEGylation methods to improve targeted gene delivery of LNPs to the lymph nodes after intramuscular administration, and to form stable LNPs for spleen targeting but with a relatively low transfection efficiency. These “findings confirm that PEGylation is critical in achieving selective organ targeting and the high in vivo transfection efficiency of LNPs (12).”
Kuo et al. reported a formulation that delivers nerve growth factor (NFG) and retinoic acid (RA) to promote the uptake of NGF and RA by induced pluripotent stem cells which accelerated the production of neurons. This could be instrumental in treating nerve injury and neurodegenerative disease (14).
These recent discoveries, along with Intellia’s recent news of the clinical trial using CRISPR as a gene-editing therapy, are incredibly exciting. The mRNA vaccines for COVID-19 were a spectacular success, and much of the credit is shared with those who committed to developing and refining lipid nanoparticles. The triumph has reignited interest in perfecting LNPs and, hopefully, further research will deliver results that are equally impactful in developing vaccines and cures for rare diseases and cancer.
References:
- Thi, Thai Thanh Hoang et al. “Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines.” Vaccines vol. 9,4 359. 8 Apr. 2021,
- Kulkarni, Jayesh A et al. “Lipid Nanoparticles Enabling Gene Therapies: From Concepts to Clinical Utility.” Nucleic acid therapeutics vol. 28,3 (2018): 146-157. doi:10.1089/nat.2018.0721
- Uchida, Satoshi et al. “Nanomedicine-Based Approaches for mRNA Delivery.” Molecular pharmaceutics vol. 17,10 (2020): 3654-3684. doi:10.1021/acs.molpharmaceut.0c00618
- Zeng C., Zhang C., Walker P.G., Dong Y. (2020) Formulation and Delivery Technologies for mRNA Vaccines. In: Current Topics in Microbiology and Immunology. Springer, Berlin, Heidelberg. //doi.org/10.1007/82_2020_217
- Scioli Montoto, Sebastián et al. “Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects.” Frontiers in molecular biosciences vol. 7 587997. 30 Oct. 2020, doi:10.3389/fmolb.2020.587997
- Kulkarni, Jayesh A et al. “Lipid Nanoparticle Technology for Clinical Translation of siRNA Therapeutics.” Accounts of chemical research vol. 52,9 (2019): 2435-2444. doi:10.1021/acs.accounts.9b00368
- Schoenmaker, Linde et al. “mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability.” International journal of pharmaceutics vol. 601 (2021): 120586. doi:10.1016/j.ijpharm.2021.120586
- Kowalski, Piotr S et al. “Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery.” Molecular therapy: the journal of the American Society of Gene Therapy vol. 27,4 (2019): 710-728. doi:10.1016/j.ymthe.2019.02.012
- Cullis, Pieter R, and Michael J Hope. “Lipid Nanoparticle Systems for Enabling Gene Therapies.” Molecular therapy: the journal of the American Society of Gene Therapy vol. 25,7 (2017): 1467-1475. doi:10.1016/j.ymthe.2017.03.013
- Cross, R. (2021, March 6). “Without these lipid shells, there would be no mRNA vaccines for COVID-19.” C&EN – Chemical & Engineering News. //cen.acs.org/pharmaceuticals/drug-delivery/Without-lipid-shells-mRNA-vaccines/99/i8.