
A native function for RAN translation and CGG repeats in regulating fragile X protein synthesis
Rodriguez CM, Wright SE, Kearse MG, Haenfler JM, Flores BN, Liu Y, Ifrim MF, Glineburg MR, Krans A, Jafar-Nejad P, Sutton MA, Bassell GJ, Parent JM, Rigo F, Barmada SJ, Todd PK.
Joint First Authors

Prof. Peter K. Todd

Ms. Shannon Wright

Dr. Caitlin Rodriguez
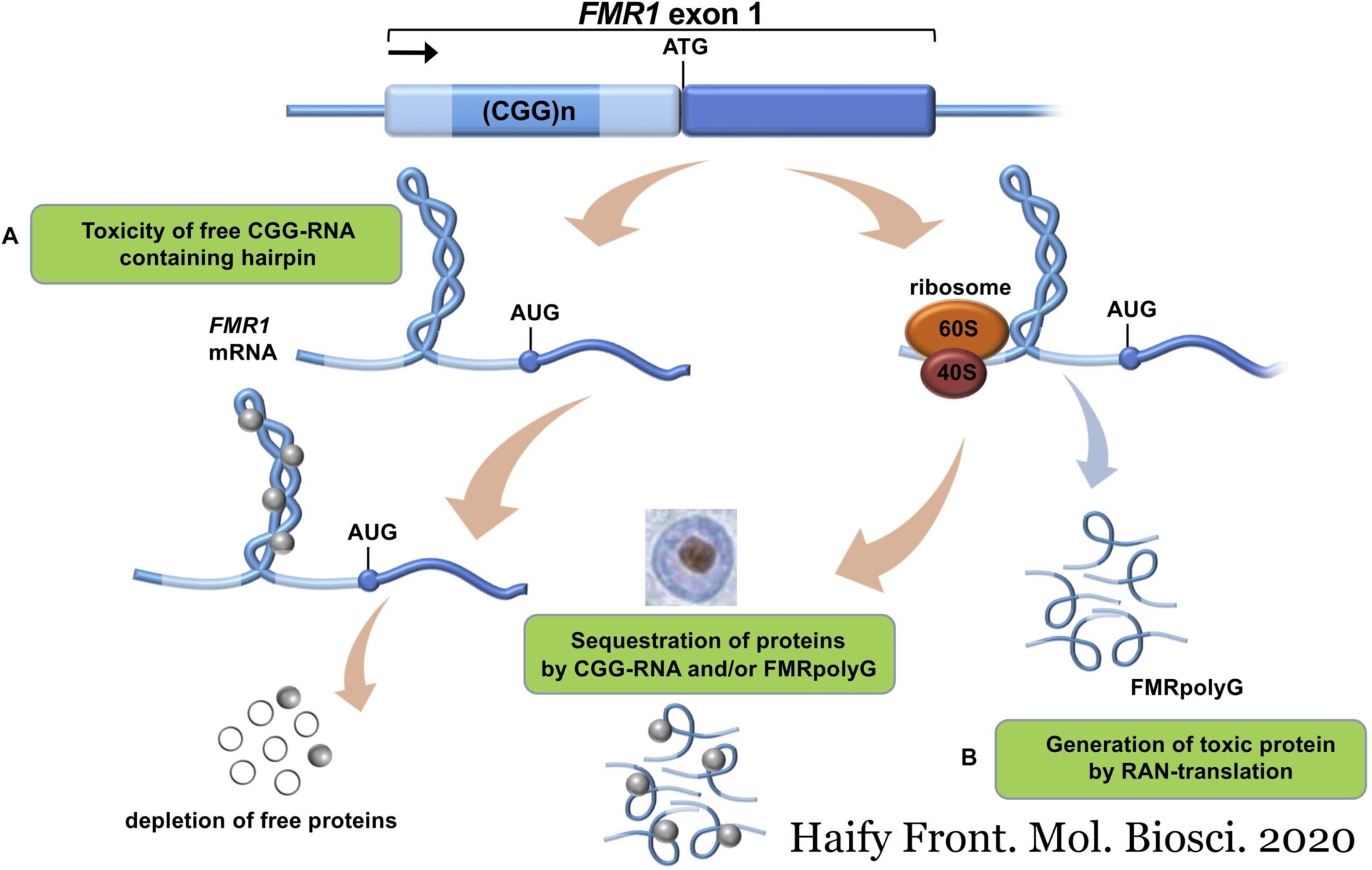
Fragile X-associated disorders are caused by the expansion of CGG repeats in the 5′-untranslated region (UTR) of FMR1. Non-AUG initiated transcripts including these CGG repeat expansions can sequester necessary RNA binding proteins and thus contribute to neuronal dysfunction. Repeat AssociatedNon-AUG translation, or RAN translation, from such transcripts can also result in toxic homopolymeric proteins, for example FMRpoly(G).Overexpression of FMRpoly(G) increases CGG repeat toxicity while reduction ameliorates it.
For more background on fragile X syndrome see here:
In this paper, Rodriguez, Wright, and colleagues investigate the physiological role of the CGG repeats and associated RAN translation in normal and repeat-expanded model systems. They found that CGG-associated RAN translation inhibits translation but not transcription of the downstream Fragile X mental retardation protein (FMRP) ORF and that RAN translation is increased in expanded repeat models.
Normally, FMRP (an RNA-binding protein) is rapidly degraded upon activation of the metabotropic glutamate receptor (mGluR), resulting in translation of previously FMRP-bound mRNAs. At the same time, mGluR activation increases translation of the FMRP mRNA, thus limiting the time FMRP-target mRNAs are free and available for translation.
The authors show that removal of normal length CGG repeats abolished this mGluR dependent feedback loop, but replacement with a non-repetitive hairpin with similar minimum free energy reconstituted it. Also, RAN translation from expanded CGG repeats decreased after mGluR activation.
Thus, the authors reasoned that downregulating CGG RAN translation should increase translation of FMRP. Indeed, steric-blocking antisense oligonucleotides targeting the RAN translation initiation codons increased FMRP expression by up to 189% in HEK293 cells and patient fibroblasts containing 69 CGG repeats. In rat neurons with 100 CGG repeats ASO treatment significantly increased survival, while in iPSC-derived human glutamatergic neurons with normal repeat length treatment increased FMRP levels by 41%. Similar results were achieved in iPSCs derived from UFM fibroblasts containing ~270 CGG repeats.
Why you should read it
Investigation of the mechanism of RAN translation led to a promising therapeutic strategy.