
Antisense oligonucleotides correct the familial dysautonomia splicing defect in IKBKAP transgenic mice
Sinha R, Kim YJ, Nomakuchi T, Sahashi K, Hua Y, Rigo F, Bennett CF, Krainer AR.
Nucleic Acids Res. 2018 Jun 1;46(10):4833-4844.
The three joint first authors:

Rahul Sinha

Young Jin Kim

Tomoki Nomakuchi
Currently, the most successful oligonucleotide therapeutic in the market is the splice modulating antisense oligonucleotide nusinersen/Sprinraza (see this previous OTS Perspective and the November 2017 Paper of the Month).
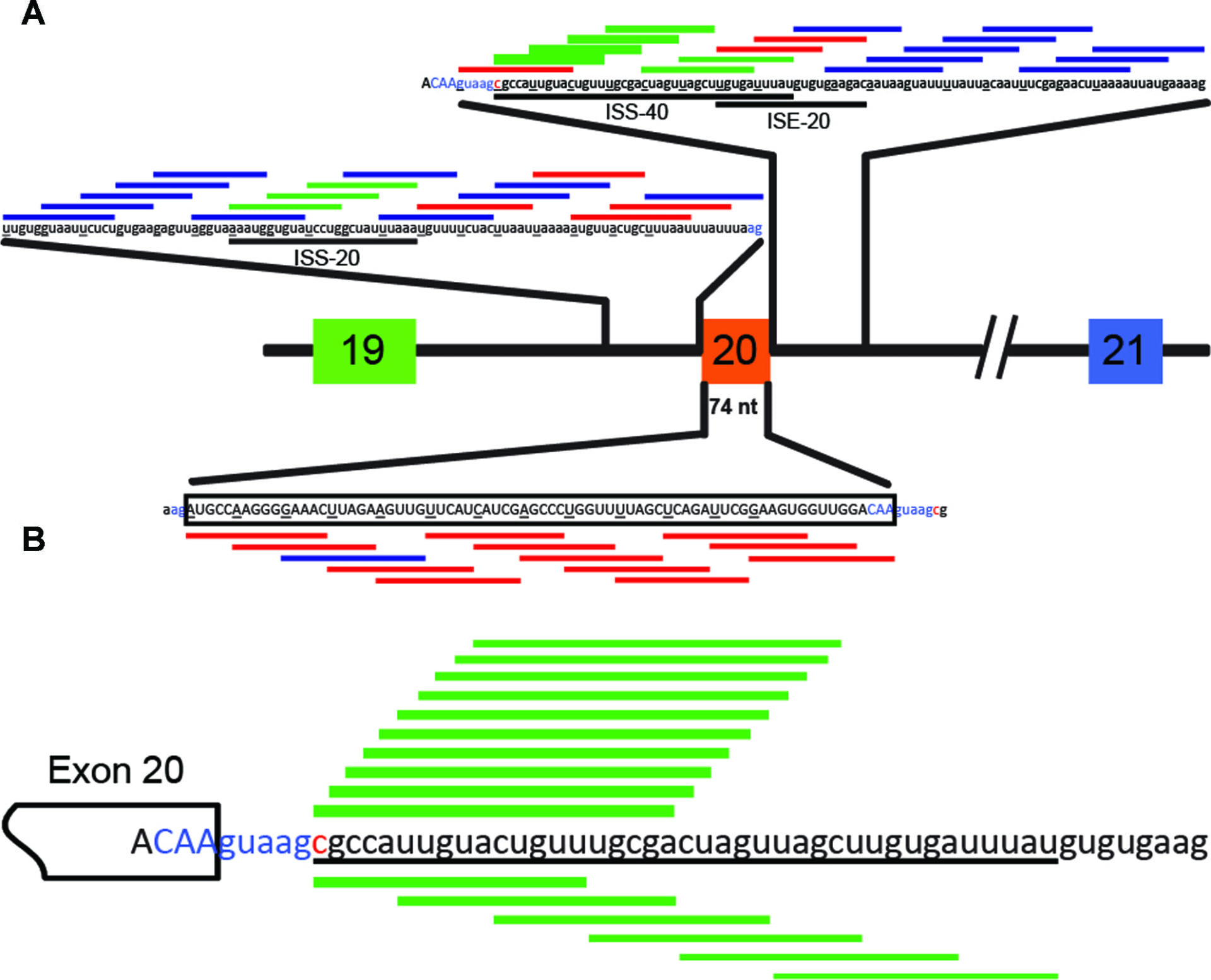
Here, Sinha and colleagues from Adrian Krainer’s lab and Ionis Pharmaceuticals, the same team that developed nusinersen, present proof of concept data for a splice modulating antisense oligonucleotide (ASO) to treat familial dysautonomia (FD). FD is an autosomal recessive inherited disease caused by a single point mutation in intron 20 (IVS20+6T→C) of the IKBKAP gene. This mutation weakens the 5′ splice site of intron 20, leading to skipping of exon 20 and a subsequent frameshift that introduces a premature termination codon in exon 21.
Watch Adrian Krainer discuss the project:
The team used minigenes to express wildtype and mutant IKBKAP gene sequences from exons 19 to 21 or 22 in HEK-293 cells and screened 49 15-mer 2-O-methoxyethylribose-phosphate ASOs, each offset by 5 bases, against the entire 74-nucleotide (nt) IKBKAP exon 20 as well as the 100-nt proximal intronic regions upstream and downstream of exon 20. This ASO walk revealed that ASOs targeting three regions (ISS-20 – upstream intron, ISS-40 and ISE-20 – downstream intron) increased exon 20 inclusion and full-length IKAP protein expression. Ten further 20-mer ASOs with a 1-nt off-set targeting the ISS-40 region were screened and oligo 7-26 almost completely restored exon 20 inclusion (up to 96%). Five nM of ASO 7-26 were sufficient to achieve similar results in patient derived skin fibroblasts.

Adrian Krainer
A 2′-O-methoxyethylribose-phosphorothioate modified version of 7-26 showed a linear dose-response of exon 20 inclusion in the brain and spinal cord of transgenic mice after intracerebroventricular (ICV) administration. These mice carry the human IKBKAP gene with the major FD mutation as well as the wild type mouse Ikbkap and as such do not demonstrate a disease phenotype. However, the mutant human transgene does show a skipping pattern similar to that of FD patients. Subcutaneous injection of the ASO also increased exon 20 inclusion in peripheral tissues. In all ASO experiments, increased exon 20 inclusion resulted in increased expression of full-length IKAP protein.
Why you should read it
This is a proof of concept study for treatment of familial dysautonomia from the team that developed nusinersen (Spinraza).






